Is Hearing Loss in Older Adults Predictive of Later Development of Dementia and Does Hearing Care Modify Dementia Risk?
“What you know, you know, what you don’t know, you don’t know.
This is true wisdom.” — Confucius.
Abstract
This paper provides an overview of the rapidly expanding research evidence-base concerning connections between hearing and cognition. It underscores the importance of distinguishing between measures to evaluate performance on various domains of cognition in healthy older adults versus measures to screen for dementia and emphasizes that correlation does not prove causation. It explains that risk at the population level is not the same as the risk for individuals and considers the evidence-based practice trio- research, clinical expertise, and patient perspectives- to guide audiologists in evaluating, integrating, and applying what we know (and do not know) to shape clinical practices. It stresses the need for an inter-professional team approach to promote healthy aging and the need to be vigilant in reading and interpreting the research so that inaccurate or misleading information will not be propagated. This snapshot of the evidence up to January 2023 ends with an outlook for many more advances and opportunities to expect in 2023.
We would all love to know the answers to these questions about hearing and cognition, but so far there is only limited evidence and we need to know what we do not know as well as what we do know. When considering changes in clinical practice, audiologists must proceed with caution, remain alert for emerging research, critically appraise emerging claims, and keep asking questions, especially when research findings are correlational. For now, one question leads to another question and then to more questions. We are compelled to ask the second question “Does hearing care modify dementia risk?” only if “yes” is the answer to the first question “Is hearing loss in older adults predictive of later development of dementia?”. To answer these questions, we must apply the principles of evidence-based practice (EBP) whereby the best currently available research evidence is integrated with clinician expertise to shape person-centered care that aligns with the values and needs of individual patients (Hickson et al., 2013; Sackett et al., 1996; Wong & Hickson, 2012). Crucially, we must strive to optimize the trio of research evidence, clinician expertise and patient perspectives (Figure 1) within a healthcare context that is rapidly evolving.
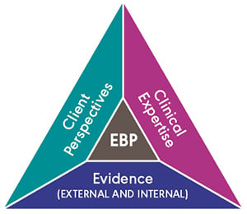
These questions bring us to new frontiers in audiology and the answers may set us on a path to redefining the role of audiologists as the healthcare system evolves to implement Integrated Care for Older People (ICOPE; WHO, 2017). The challenge for audiologists will be to deliver hearing care that promotes healthy aging (see WHO definition; WHO 2020), including for people at risk for or living with dementia (Wittich et al., 2022). Audiologists providing hearing healthcare to older adults must prepare to shift their practices away from working mostly as siloed specialists tethered to sound-booths towards a new era of practice working in inter-professional teams in new contexts (e.g., Wallhagen et al., 2021). Innovations are expected in contexts spanning inter-professional primary care, home care and long-term care (LTC). The impending evolution of practice can only be successful if it is supported by new research, new clinical training, and new person-centered approaches to integrated care. Since 2020, there has been an explosion in new research. There is much to look forward to in 2023 as more research is published and new ideas are incubated concerning new clinical training and new ways to implement person-centered hearing care for older adults.
“WHO defines healthy ageing as ‘the process of developing and maintaining the functional ability that enables wellbeing in older age.’ Functional ability is about having the capabilities that enable all people to be and do what they have reason to value. This includes a person’s ability to: meet their basic needs; learn, grow and make decisions; be mobile; build and maintain relationships; and contribute to society.” World Health Organization (WHO, 2020).
Question 1: Is hearing loss in older adults predictive of later development of dementia?
What do we need to ask?
The first question is about prognosis. For such questions, the best evidence will require large-scale longitudinal studies to determine if early conditions develop (or not) into later conditions (Sackett et al., 1996). If a correlation is found between an early condition (e.g., hearing loss) and a later condition (e.g., dementia), then possible mechanisms explaining the correlation must be explored before any conclusions can be made about whether or not the first condition causes the second condition. Correlations do not prove causation. If the observed association between the early and late conditions is explained simply by both conditions resulting from some other common cause (e.g., declines in cardiovascular health), then preventing or treating the common cause could prevent both conditions. Follow-up research would be needed to determine whether or not treating a common cause (e.g., by increasing physical activity or stopping smoking to address cardiovascular health) could reduce risk or slow the progress of both conditions. Alternatively, if causal mechanisms are identified, then knowledge of the causal mechanisms could inform treatment such that treatment would address the first condition, but also aim to reduce risk for or delay the second condition.
Further research would be needed to determine if social determinants of health (e.g., sex, age, race, education, occupation) or comorbid health conditions (e.g., vision loss, depression, loneliness, obesity) may also be associated with the second condition. More follow-up questions would ask whether or not these factors or others (e.g., stigma, social support) could also be causally linked to the later condition and if there are interactions such that multiple conditions amplify or mediate the risk that the first condition (hearing loss) poses for the second condition (dementia). Of course, it is also possible that there are many variations on the answers to these questions that depend on the specifics of the first condition (e.g., age of onset, duration, degree, type and etiology of hearing loss) and/or the specifics of the second condition (e.g., the type and/or stage of dementia).
What do we know (or not) now?
Sensory-cognitive associations in cognitively healthy older adults.
Evidence does support an association between hearing loss and performance on cognitive tests in older adults. Sensory-cognitive associations have been recognized for at least six decades (e.g., Birren et al., 1963). Hypotheses that might explain age-related sensory-cognitive links were proposed in seminal reports of findings from the Berlin Aging Study published over 25 years ago (Lindenberger & Baltes, 1994; Baltes & Lindenberger, 1997). The cross-sectional results from Berlin indicated that variance in hearing and visual acuity accounted for 93% of the age-related variance in cognitive measures (processing speed, reasoning, memory, knowledge, and fluency) and that the strength of the sensory-cognitive associations increased with age. Over the intervening quarter century, there has been an exponential increase in research, including analyses of data from longitudinal studies, to test hypotheses regarding the links between hearing and cognition, as well as links between vision and cognition. Research on sensory-cognitive associations in older adults continues to be driven by the same core hypotheses, including the common cause hypothesis and hypotheses about short-term and/or long-term causal effects of auditory aging on cognitive aging (for a review see Whitson et al. 2018). Many studies have replicated the findings of sensory-cognitive links, including a study conducted with data from the Canadian Longitudinal Study of Aging (Phillips et al., 2022). One systematic review and meta-analysis of 40 studies from 12 countries with a total of over 20,000 unique participants found small but significant associations between audiometric threshold measures and measures of ten cognitive domains (Loughrey et al., 2018). Other reviews have summarized evidence of associations between supra-threshold measures of auditory processing and measures of cognition (Panza et al., 2015a,b; Panza et al., 2018a,b; Yuan et al., 2018). However, despite the evidence that there are age-related sensory-cognitive associations, the underlying mechanisms are still unknown and it is still disputed whether sensory declines and cognitive declines simply have a common cause or if sensory decline causes cognitive declines in the short-term (due to degradation of sensory information) and/or in the long-term (due to sensory deprivation). Importantly, significant associations between hearing and performance on numerous measures of cognitive processing in healthy aging samples does not mean that hearing loss is necessarily predictive of the later development of clinically significant cognitive impairment or dementia.
Sensory-cognitive associations in cognitively impaired older adults.
An excellent critical review of the literature recently concluded that “Research has yet to determine if there is an independent effect of hearing loss on cognitive impairment for older adults.” (Powell et al., 2021, pg 396). This point was reinforced in a November 2022 report of findings from the Mayo Clinic Study of Aging (Marinelli et al., 2022). Notably, using the data of 1200 participants (the subset who had completed hearing assessments in the audiology clinic), significant associations were found between pure-tone thresholds and performance on cognitive tests (memory, attention/executive function, language, and visuospatial skills), but not between pure-tone thresholds and incident dementia, whether or not participants used hearing aids (17% of the sample developed dementia over seven years in this prospective longitudinal study of older adults). Curiously, informant-based measures of hearing difficulties were associated with the development of dementia even though pure-tone thresholds were not. A commentary on the study (Loughrey, 2022, pg e805) noted that “Age-related hearing loss and dementia are complex disorders and the mechanistic basis underlying their potential association has not yet been clarified.” Loughrey suggested that more research is needed to examine associations between different measures of hearing (pure-tone thresholds, supra-threshold auditory processing measures and/or self-report or other-report measures) and various types of dementia at different stages and in more diverse populations. Some researchers (Eberhard et al., 2022; Vaden et al., 2022) have begun looking more closely at how different phenotypes of age-related hearing loss (e.g., sensory vs. metabolic subtypes as indexed by audiometric configurations) are associated with domain-specific cognitive scores on dementia screening tests in older adults with varying degrees of cognitive loss. Furthermore, apart from audiometric thresholds, central auditory processing dysfunction has also been hypothesized to be an early marker of dementia (e.g., Gates et al., 1996, Gates et al., 2011). A better understanding of the interplay among age-related cochlear and central auditory declines, cognitive declines, and dementia will be essential for the prevention or early identification of subgroups at greater risk.
Associations between auditory and cognitive measures vs. functioning in everyday life.
In everyday life, the entire auditory nervous system from cochlea to cortex is involved, along with other sensory systems, as listeners engage in bottom-up processing to relay incoming signals to the brain and top-down processing whereby the brain controls attention to incoming signals, matches them with knowledge stored in memory, and coordinates behavioral responses based on interpretations of multi-sensory information. It is noteworthy that the Mayo Clinic study used the sub-sample of participants who had been seen in the audiology clinic. Clinical samples may differ in important ways from community samples. For example, self-report measures of hearing difficulties have been shown to differ between community samples and samples of older adults drawn from audiology clinics and between those drawn from clinical samples who do or do not use hearing aids (Humes & Dubno, 2021). The intriguing finding of a significant association between informant-based measures of hearing difficulty and dementia in the Mayo clinical sample points to the importance of functioning in everyday life. Studying statistical associations between isolated auditory and cognitive measures will likely not be sufficient to uncover if and how gradual age-related changes in the auditory system alter patterns of brain activity as listeners engage in complex everyday activities (Pichora-Fuller, 2020; Pichora-Fuller et al., 2017; Pichora-Fuller & Singh, 2006). Neuroimaging studies of brain structures and networks in animals (e.g., Glennon et al., 2022; Shilling-Scrivo et al., 2022) and humans (e.g., Giroud et al., 2021; Peelle, 2018) are beginning to shed light on age-related changes in auditory-cognitive networks. Discovery of the mechanistic bases of sensory-cognitive associations and age-related changes in the complex interplay of sensory-cognitive brain networks during everyday functioning will have important implications for rehabilitative interventions designed to promote healthy aging for older adults who vary in hearing and cognitive health.
Question 2: Does hearing care modify dementia risk?
What do we need to ask?
The second question is about intervention. For such questions, the best evidence will come from research to evaluate the effectiveness of a treatment in comparison to providing no treatment or alternative treatments. Large-scale prospective studies using randomized assignment of participants to different treatment conditions (i.e., randomized control trials (RCTs)) are considered to provide one of the strongest types of evidence. Comparisons between conditions are needed because it is important to prove that doing something is better than doing nothing, and if there are multiple options, then it is important to determine which of the alternatives is best. In addition, randomizing participants to treatment options is necessary to rule out the possible effects of self-selection (e.g., those in higher socio-economic positions seek treatment, and those with lower socio-economic positions do not seek it; those with less stigma and/or more functional difficulties in everyday life use hearing aids compared to those who do not). Beyond learning about findings, it is important to be able to evaluate the strengths and weaknesses of the research that was conducted. What the findings tell us (or not) depends on the research design. The PICO (population, intervention, comparison, outcome) framework is useful for designing and also understanding intervention research, including what a study can tell us about a particular target population, a method of implementing the intervention, the comparison of inactive and/or active control conditions tested, and the measures used to evaluate outcomes (see ASHA online resources https://www.asha.org/research/ebp/frame-your-clinical-question/). The PICO framework is helpful when reviewing multiple research studies, especially when differences in the findings reported across studies may be explained by differences in the study designs. The PICO framework is also helpful for understanding how generalizable research findings may be to particular practice settings. Systematic reviews or meta-analyses of RCTs provide valuable information about the effectiveness of interventions and their generalizability. Nevertheless, while evidence based on research is important, it is only one component of evidence-based practice. Successful implementation of treatments into practice will depend on the strength and generalizability of research findings as well as the clinician’s expertise and the patient’s perspectives and choices in the context of the practice setting.
What do we know (or not) now?
Is hearing loss a modifiable risk factor for dementia?
Hearing loss was reported to be the greatest potentially modifiable risk factor for dementia in a review paper published in the Lancet in 2017 (Livingston et al., 2017). In the 2020 update of the Lancet review paper (Livingston et al., 2020), twelve potentially modifiable risk factors were identified (three more than had been identified in the 2017 review), including lower educational attainment, hypertension, smoking, obesity, depression, physical inactivity, diabetes, infrequent social contact, excessive alcohol consumption, head injury, air pollution, and hearing loss. As more evidence becomes available, it is expected that additional risk factors, such as vision loss (Cao et al., 2022; Ehrlich et al., 2022), will be added in the next update. Audiologists need to understand the claims made (or not) in these frequently cited reviews: 1. Hearing loss is only one of a growing number of potentially modifiable risk factors and hearing loss may combine with other factors; 2. These risk factors are POTENTIALLY modifiable, but it is not proven that they can ACTUALLY modify dementia risk; 3. The percentages reported reflect the population-attributable risk fractions (PAFs), and the disproportionate high weight attributed to hearing loss is due, in part, to the high prevalence of hearing loss in the older adult population (i.e., the percentage of risk is calculated at the population level and is not an estimate of the risk of individuals). Risk at the individual level will depend on many factors, including social determinants of health, comorbid health conditions, and supports for or barriers to healthy aging in an individual’s social and physical environments.
Social determinants of health matter. There are variations in PAFs of risks for dementia across low- and middle-income countries (Mukadam et al., 2019). Within countries, race and ethnicity contribute to variations in PAFs for dementia risk, including in the USA (Lee et al., 2022), the United Kingdom (Mukadam et al., 2022), and New Zealand (Ma’u et al., 2021). In addition to race and ethnicity, sex has been shown to contribute to variations in risk factors in the USA (Nianogo et al., 2022). Analyses of Canadian data also suggest sex-specific differences in sensory-cognitive associations in cognitively healthy (Al-Yawer et al., 2022a) and cognitively impaired samples (Al-Yawer et al., 2022b). The importance of taking regional differences and the social determinants of health (e.g., race, ethnicity, sex) into account raises yet more questions about how hearing healthcare should evolve to address social inequities in healthcare systems (McMahon, 2021). In clinical practice, these variations in risk may influence how audiologists could best tailor person-centered interventions for individuals with hearing loss who may be at risk for or be living with dementia (Hoffmann et al., 2022).
Comorbidities matter. Integrated person-centered care for older adults will need to be informed by co-morbid health conditions that interact with hearing loss to affect healthy aging and functioning in everyday life. ICOPE (WHO, 2017; Thiyagarajan et al., 2019) provides guidelines for integrated care to optimize functioning by addressing six capacities: hearing, vision, mobility, cognition, depression, and nutrition. These and other comorbidities may also interact with hearing loss to affect the risk of dementia. Indeed, aging-related declines across all sensory and motor systems seem to be associated with Alzheimer’s disease (Albers et al., 2015). The Lancet review (Livingston et al., 2020) identified twelve potentially modifiable factors for dementia. Clearly, some of these risk factors for dementia are also risk factors for hearing loss (e.g., hypertension, diabetes), and hearing loss can increase the risk of some of the other risk factors for dementia (e.g., social isolation, depression) (for a review see Pichora-Fuller et al., 2015). Given the importance of vision for communication, it is particularly important for audiologic rehabilitation (AR) that vision is already one of the capacities identified for ICOPE and that vision may soon be added to the Lancet list of risk factors for dementia. Analyses of data from healthy Canadians show that sensory difficulties were reported more often by males and by those with more comorbid health conditions and that hearing loss and vision loss can have combined effects on cognitive performance (Phillips et al., 2022); compared to those who did not report vision difficulties, those who did report them were more likely to also report hearing difficulties and vice versa (Hämäläinen et al., 2021). There is also mounting evidence that dual sensory loss poses more risk for dementia than either hearing loss alone or vision loss alone (Huang et al., 2022).
Does the use of hearing aids and/or cochlear implants modify risk for dementia?
A systematic review and meta-analysis (Yeo et al. 2022) reported promising findings but concluded that randomized control trials are needed to provide stronger evidence. The review considered 31 studies (25 observational studies, 6 non-RCT trials) with 137,484 participants, and quantitative analyses were conducted using 19 (15 observational studies, 4 non-RCT trials). Meta-analysis of 8 studies with follow-up durations ranging from 2 to 25 years showed significantly lower hazards of any cognitive decline among hearing aid users compared with participants with uncorrected hearing loss, and a meta-analysis of 11 studies examining short-term effects found a 3% improvement in cognitive test scores after the use of hearing aids. A published comment on the study (Denham et al., 2022) noted that significant benefits of device use were found when data across studies were aggregated in the meta-analyses but that the findings of most individual studies did not reach significance. It was also noted that the meta-analyses included participants with normal cognition and participants with mild cognitive impairment at baseline such that it is impossible to determine if the benefits differed depending on baseline cognitive status. Similarly, the associations of hearing loss with dementia vary with the degree of hearing loss; for example, a 2023 research letter in JAMA (Huang et al., 2023) reported analyses of data from a nationally representative sample in the USA showing that an association between hearing aid use and dementia was found only for those with moderate-severe hearing loss. In summary, the main limitations of the evidence captured in the review by Yeo and colleagues (2022) were minimal characterization of hearing loss, lack of measures of specific cognitive domains, and inadequate control for possible confounding factors (e.g., social determinants of health and comorbidities).
Despite the promise offered by research to date, there are still unanswered PICO questions about how the results may depend on (P) the populations studied (degree of hearing loss, cognitive status at baseline, social determinants of health), (I) the interventions administered (hearing aid or cochlear implant and what other rehabilitation components were provided), (C) the lack of randomization of participants to comparison groups with/without devices and the lack of control for confounding factors such as comorbidities, and (O) the outcome measures used to evaluate cognition and the time over which change was evaluated. It is not surprising that the use of devices would have immediate effects on many cognitive measures simply because the use of devices would improve audibility and the quality of sound inputs, thereby reducing the effects of information degradation on cognitive processing, especially on auditory memory items that typically drive scores on the most commonly used general cognitive screenings tests (Al-Yawer et al., 2019; Dupuis et al., 2015; Eberhard et al., 2022). Such short-term benefits may or may not result in longer term changes by staving off the effects of auditory deprivation on alterations in cognitive processing and patterns of brain activation. Some of these questions may be answered in 2023 when it is expected that the results of the Aging and Cognitive Health Evaluation in Elders (ACHIEVE; NCT03243422) RCT will begin to be published (Deal et al., 2018). In the meantime, audiologists should be aware of the WHO (2019) recommendations regarding interventions to reduce dementia. Strong recommendations for physical activity and tobacco cessation were made based on a review of the effectiveness of interventions to reduce dementia risk. It was concluded that there was insufficient evidence to recommend use of hearing aids to reduce the risk of cognitive decline and/or dementia, but that hearing screening followed by provision of hearing aids should be offered to older people for timely identification and management of hearing loss to improve communication and functioning as recommended in the WHO ICOPE guidelines. Questions about screening arise from the recognized need for timely identification and management of hearing loss.
Should hearing and/or cognition be screened in older adults?
Hearing screening. The WHO World Report on Hearing (2021a,b) recommends universal hearing screening conducted at regular health checks for older adults beginning at age 50. In the wake of the Lancet papers by Livingston and her colleagues (2017, 2020) and the WHO (2019a,b) recommendations, geriatric guidelines for the diagnosis and treatment of dementia in primary care have begun to recommend that hearing be screened and hearing care provided as indicated to optimize the functioning of older adults at risk for or living with dementia (for Canadian guidelines see Ismail et al., 2020). Canadian research has demonstrated the feasibility of hearing screening in memory clinics (Dupuis et al., 2019a) and emerging models for collaborations between audiologists, neuropsychologists and physicians in inter-professional geriatric teams (Reed et al., 2022). International Practice Recommendations for the Recognition and Management of Hearing and Vision Impairment in People with Dementia (Littlejohn et al., 2022) have set the stage for many new initiatives, including inter-professional training to develop clinical expertise amongst team members. However, much more work is needed to integrate hearing care into inter-professional primary care for older adults starting when they have normal cognition and as they may progress to living with dementia.
Cognitive screening. Across countries, including Canada (Ismail et al., 2020), the USA (Owens et al., 2020), and Brazil (Smid et al., 2022), current geriatric guidelines for dementia care do NOT recommend cognitive screening for asymptomatic individuals. In an editorial comment on the USA guidelines, Brayne (2020) cites UK guidelines stating that screening programs should balance clinical benefits with the potential for harm in the context of effective resource allocation; she argues that cognitive screening should not be assumed to be beneficial because there may be severe counter-balancing harms insofar as many of those who may categorize as having mild cognitive impairment will not develop dementia (i.e., predictive value is poor). Furthermore, there are no effective treatments to prevent or cure dementia other than health-promoting lifestyle recommendations (e.g., physical activity, smoking cessation, diet) that could be made without needing to screen cognition. In another editorial, Petersen and Yaffe (2020) suggest that it is important for patients to appreciate whether or not their own or others’ concerns over their cognitive function warrant investigation. Thus, cognitive screening may be indicated if symptoms and concerns are reported by the individual and/or their significant other(s), but not for those who have no symptoms or concerns. Also, cognitive screening would be unnecessary for people who have already been diagnosed with cognitive impairment or dementia.
When taking a general health history, along with questions about other relevant health conditions (e.g., vision, dexterity), it seems reasonable for audiologists to ask patients and/or their significant others if they have concerns about cognition (memory, attention, thinking) or if they have ever been tested for or diagnosed with a cognitive impairment. Cognitive screening results may already be available in electronic medical records. If cognitive loss is a concern and has not been tested already, it may be appropriate to conduct a cognitive screening test if the audiologist and the patient have a clear idea about how the test results will be used to inform care planning. Many cognitive screening tests may be chosen depending on patient characteristics (e.g., education, culture, language) and clinical context (for an overview of these choices, see the webinar by Ismail, 2021). Performance on screening tests may be influenced by myriad factors, including physical (e.g., fatigue, pain) and mental (e.g., mood) health issues. Testing itself may produce stress (Lupien et al., 2013) and induce ageist stereotype threats (Chasteen et al., 2005) that could also affect performance. Notably, for those with hearing and/or vision loss, care must be taken to ensure that stimuli are optimally presented in optimal environments to reduce over-estimation of cognitive loss (Dupuis et al., 2015, 2016). However, standards do not exist for sensory assessment and sensory accommodation during cognitive assessment (Liu et al., 2022).
Of course, the psychometric properties of the screening test, including test sensitivity and specificity for correctly categorizing passes and failures, are essential for evaluating the comparative effectiveness of test options. It is worth noting that one recently developed cognitive screening test, Cognivue®, has been heavily marketed to audiologists, including presentations (e.g., Presley, 2022) sponsored by the company and offered by AudiologyOnline (2022) that have been approved for continuing education by Canadian professional associations including the Canadian Academy of Audiology (CAA), Speech-Language Audiology Canada (SAC) and the Association of Hearing Instrument Practitioners of Ontario (AHIP). Importantly, however, misleading marketing claims have been made in these promotional materials and presentations, including claims about the effectiveness of the test based on a study that did not use standard methods for assessing screening tests (Cahn-Hidalgo et al., 2020). An independent investigation found the Cognivue test to be inferior to the Montreal Cognitive Assessment (Nasreddine et al., 2005), advised that further evidence was needed to demonstrate that the test meets validity and reliability standards, and warned that “misdiagnosing of neurocognitive disorders can pose unnecessary psychological and emotional harm to patients and their families and also lead to incorrect treatment and undue healthcare costs” (Rose et al., 2021, pg 265). In Canada, an online cognitive screening test developed at Baycrest in Toronto, Cogniciti (https://cogniciti.com/), is available for free to anyone who wishes to self-screen and access resource materials and information about brain health. A link to Cogniciti is available on the Baycrest Audiology website. Providing patients with a free cognitive self-screening option with supporting information and resources concerning brain health seems to address the matter raised by Petersen and Yaffe (2020) regarding cognitive screening in response to patients’ concerns about whether or not they have clinically significant cognitive impairment or dementia. Furthermore, the Cogniciti self-screening option can be taken at the convenience of the individual, and it short-circuits involving the audiologist in administering the test and making referrals for those who fail screening. It also safeguards against the possibility of unethical practice given the risk that cognitive screening may foster misleading inferences about the connections between hearing loss and dementia that could inappropriately influence patient decisions such as hearing aid purchase.
How could cognitive tests be used in evidence-based hearing care?
It is important to distinguish between cognitive tests to screen for cognitive impairment or dementia vs. cognitive tests to evaluate the cognitive abilities of healthy older adults that are involved in successful communication functioning in everyday life (Phillips, 2016). Based on the evidence reviewed above, cognitive screening tests raise issues related to the clinical expertise and patient perspectives components of EBP. Most importantly, cognitive screening tests do not necessarily provide measures of cognitive abilities that would be useful to inform AR.
Inter-professional clinical expertise and ethics upholding patient values. Audiologists are expected to adopt EBP based on the best research evidence. Crucially, EBP must be aligned with clinical expertise and the patient’s perspectives. Some non-peer-reviewed papers in publications for hearing professionals (e.g., Nalley, 2021) have floated questionable suggestions about cognitive screening by audiologists, including the idea that a new role for audiologists is to act as the gatekeepers to cognitive care and the idea that cognitive screening is a way to boost sales. Aspirations to position audiologists as gatekeepers or managers of cognitive care seem unrealistic, unethical given the limited competency of most audiologists to provide cognitive care, and contrary to proposals for inter-professional teamwork as outlined in the international practice recommendations for the recognition and management of hearing and vision impairment in people with dementia (Littlejohn et al., 2022). Effective inter-professional teams will rely on audiologists and other health professionals learning about each other and developing new ways of working collaboratively in the circle of care (Dupuis et al., 2019b). Inter-professional team collaborations seem necessary to provide the coverage of competencies needed to satisfy the clinical expertise component of evidence-based integrated person-centred care for older adults with sensory and cognitive health issues.
The suggestion of leveraging the discussion of cognitive decline to promote hearing aid sales raises serious concerns about ethics (Blustein et al., 2020). Risks arising from misleading information pertain to the patient perspective component of EBP. The College of Audiologists and Speech-Language Pathologists of Ontario (CASLPO) put new standards for advertising into effect in December 2022. The standard's rationale states, “Patients must be able to trust that audiologists and speech-language pathologists will engage in and be accountable for truthful, ethical, and professional advertising. The public reasonably expects that a regulated health professional’s advertising will not be deceptive, pressure-based, or promote unnecessary services.” The first standard states, "Advertising must be accurate and factual and not contain misleading or unverifiable information.” (CASLPO, 2022). The public’s lack of trust in hearing healthcare professionals is a barrier to help-seeking for hearing problems (McMahon et al., 2021). Providing inaccurate or misleading information about auditory-cognitive associations in aging is likely to further jeopardize trust and potentially violate ethical standards. Insofar as cognitive screening of asymptomatic individuals contravenes the recommendations of geriatric guidelines, it seems that cognitive screening of asymptomatic adults seen in audiology would be an example of promoting an unnecessary service. The use of misleading information by suggesting that buying a hearing aid is beneficial for preserving cognitive health has already been disciplined by the College of Speech and Hearing Professionals of British Columbia (CSHBC, 2019), with the Committee of Inquiry concluding that “In the Committee’s view, these materials suggested or may have caused a member of the public to believe that there is a causal link between hearing loss and the onset of dementia, or that the use of hearing aids could prevent the onset of dementia.” The risks of misinformation for public health have been exacerbated by spreading non-peer-reviewed claims and distortions of peer-reviewed evidence in social media and marketing. Some of this misinformation has even been disseminated through continuing education courses approved by our own associations without these courses being properly scrutinized using an EBP lens. The risks of misinformation to public health are now being combatted through efforts such as the ScienceupFirst (https://www.scienceupfirst.com/) initiative of the Canadian Association of Science Centres. Canadian audiologists and the professional associations representing them must be more vigilant not to propagate misleading information, and they should be more proactive in engaging with researchers to ensure that EBP is supported by the best available peer-reviewed research evidence.
Cognitive tests to inform AR for cognitively healthy older adults. Some older adults are experienced hearing aid or cochlear implant users, but they may have questions about healthy aging because they have read media headlines about hearing loss being associated with increased risk for many health issues, including dementia. These patients should be counselled and informed of the WHO (2019) recommendations for lifestyle behaviours that could be beneficial in reducing dementia risk. Whether older adults are experienced device users or have their first AR assessment, there are well-known measures that audiologists can use to evaluate how cognitive abilities may contribute to communication functioning in everyday life. Considerable research has examined the cognitive skills that contribute to speech understanding (e.g., Humes et al. 2013), and chapters on AR for older adults (e.g., Humes et al., 2020; Pichora-Fuller, 2021) cover how cognition can be measured using available audiological tools (e.g., questionnaires, speech-in-noise tests, binaural tests, temporal processing tests, auditory memory tests) and how cognitive abilities can be used to inform AR following the framework of the WHO International Classification of Functioning, Disability and Health (ICF; WHO, 2001). It is beyond the scope of this paper to elaborate further, but audiologists need to realize that there are many existing resources on this topic.
Cognitive tests to inform AR for people living with dementia. Cognitive screening tests are not diagnostic. AR considerations for patients diagnosed with cognitive impairment or dementia have been developed and summarised in review papers (e.g., Dawes et al., 2022; Pichora-Fuller et al., 2013). Importantly, there is active research on implementing integrated person-centered care for older adults with combined hearing, vision, and cognitive impairments (Leroi et al., 2022). Scaling future directions for AR will necessitate that training on dementia becomes more widely available to audiologists and that training about hearing loss becomes more widely available to other health professionals in the circle of care. Furthermore, new inter-professional training options should be co-constructed with people living with dementia. University-provided free courses on dementia for health professionals are available from the Wicking Research and Dementia Education Centre at the University of Tasmania in Australia (https://www.utas.edu.au/wicking/understanding-dementia). In Canada, the Canadian Dementia Learning and Resource Network (https://cdlrn.the-ria.ca/news/ria-receives-2m-in-federal-funding-to-lead-national-dementia-projects/) based at the University of Waterloo has received funding from the Public Health Agency of Canada and the Alzheimer’s Society to develop training on dementia for health professionals and the development of a community of practice to enable projects from across the country to share information and collaborate to help inform dementia policy and practice in Canada.
What should we do in 2023?
Much evidence has accumulated over previous decades, with a remarkable acceleration of research since 2020, a flood of publications in 2022, and the first 2023 papers on this topic already published. As we embark on 2023, we can look forward to the publication of important reports, including the first findings from the ACHIEVE RCT (Deal et al., 2018), an update of the Lancet review by Livingston and colleagues, and an update of guidelines following the 2023 Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Audiologists can seek opportunities for high-quality, evidence-based continuing education, including a presentation by Natalie Phillips at the Conference of the Canadian Academy of Audiology in Ottawa in October. Audiologists will be welcomed to join the Canadian Dementia Learning and Resource Network Community of Practice. In 2023, Canadian audiologists should plan to attend the World Congress of Audiology in Paris in 2024 (https://wca2024paris.com/), including the Round Table on aging and cognition. In 2023, the International Society of Audiology (ISA; https://isa-audiology.org/) will launch a community of practice to promote sharing of information about hearing care for older adults across settings from primary care to homecare to LTC. In 2023, the ISA, with the International Collegium of Rehabilitative Audiology (ICRA; https://icra-audiology.org/), will participate in the World Rehabilitation Alliance (WHO, 2022), including contributing to the working stream concerned with inter-professional teams in primary care. ISA and ICRA will work with the International Federation of Ageing (https://ifa.ngo/) to advance hearing care during the UN Decade of Health Aging (https://www.who.int/initiatives/decade-of-healthy-ageing). We should all keep asking questions, seeking answers, and thinking about what we know and don’t know (yet)!
References
- Albers, M. W., Gilmore, G. C., Kaye, J., Murphy, C., Wingfield, A., Bennett, D. A., et al. (2015). At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s and Dementia, 11(1), 70–98. http://dx.doi.org/10.1016/j.jalz.2014.04.514
- Al-Yawer, F., Bruce, H., Li, K., Pichora-Fuller, M. K., & Phillips, N. A. (2022a). Sex-related differences in the associations between Montreal Cognitive Assessment scores and pure-tone measures of hearing. American Journal of Audiology, 31(1), 220–227. https://doi.org/10.1044/2021_AJA-21-00131.
- Al-Yawer, F.,Pichora-Fuller, M.K., & Phillips, N. (2019). The Montreal Cognitive Assessment (MoCA) after omission of hearing-dependent subtests: Psychometrics and clinical recommendations. Journal of the American Geriatrics Society, 67, 1689-1694. DO https://doi.org/10.1111/jgs.15940
- Al-Yawer, F., Pichora-Fuller, M. K., Wittich, W., Mick, P., Giroud, N., Rehan, S., & Phillips, N. (2022b). Sex-specific interactions between hearing and memory in older adults with mild cognitive impairment: Findings from the COMPASS-ND Study. Ear and Hearing, PAP December 29, 2022. | DOI: 10.1097/AUD.0000000000001322
- AudiologyOnline. (2022). Industry Innovations Summit. CEU’s approved by CAA, SAC, and AHIP:https://www.audiologyonline.com/audiology-ceus/course/cognition-and-audition-supporting-evidence-37381; Cognivue advertising leading Continuing Education listing https://www.audiologyonline.com/partners/cognivue/
- Baltes, P., & Lindenberger, U. (1997). Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging, 12(3), 410–432. https://doi.org/10.1037//0882-7974.12
- Birren, J. E., Botwinick, J., Weiss, A. D., & Morrison, D. F. (1963). Interrelations of mental and perceptual tests given to healthy elderly men. In Human aging: a biological and behavioural study (pp. 143–156). Washington, DC: Government Printing Office. https://doi.org/10.1037/10776-010
- Blustein, J., Weinstein, B. E., & Chodosh, J. (2020). Marketing claims about using hearing aids to forestall or prevent dementia. JAMA Otolaryngol Head Neck Surg.,146(8), 765–766. https://doi.org/10.1001/jamaoto.2020.0854
- Brayne C. (2020). Evidence still lacking for recommendation of screening for cognitive impairment in older adults. JAMA Intern Med., 180(4), 483-484. https://doi.org/10.1001/jamainternmed.2019.7522
- Cahn-Hidalgo, D., Estes, P. W., & Benabou R. (2020). Validity, reliability, and psychometric properties of a computerized, cognitive assessment test (Cognivue®). World J Psychiatry, 10(1), 1-11. https://www.wjgnet.com/2220-3206/full/v10/i1/1.htm
- Cao, G. Y., Chen, Z. S., Yao, S. S., Wang, K., Huang, Z. T., Su, H. X., Luo, Y., De Fries, C. M., Hu, Y. H., & Xu, B. (2022). The association between vision impairment and cognitive outcomes in older adults: a systematic review and meta-analysis. Aging & mental health, 1–7. Advance online publication. https://doi.org/10.1080/13607863.2022.2077303
- CASLPO (College of Audiologists and Speech-Language Pathologists of Ontario). (2022). Advertising Standards (approved December 2, 2022; effective December 13, 2022). https://caslpo.com/sites/default/uploads/files/PS_EN_Advertising_Standards.pdf
- Chasteen, A. L., Bhattacharyya, S., Horhota, M., Tam, R., & Hasher, L. (2005). How feelings of stereotype threat influence older adults’ memory performance. Experimental Aging Research, 31(3), 235–260. https://doi.org/10.1080/03610730590948177
- CSHBC (College of Speech and Hearing Health Professionals of British Columbia). (2019). Public notice on action and resolution of inquiry. https://cshbc.ca/public_notices/c-marke-hambley-rhip/
- Dawes, P., Littlejohn, J., Bott, A., Brennan, S., Burrow, S., Hopper, T., & Scanlan, E. (2022). Hearing assessment and rehabilitation for people living with dementia. Ear and Hearing, 43(4), 1089–1102. https://doi.org/10.1097/AUD.0000000000001174
- Deal, J. A., Goman, A. M., Albert, M. S., Arnold, M. L., Burgard, S., Chisolm, T. et al. (2018) Hearing treatment for reducing cognitive decline: design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimer’s Dementia Transl Res Clin Interv, 4, 499–507. https://doi.org/10.1016/j.trci.2018.08.007
- Denham, M. W., Weitzman, R. E., & Golub, J. S. (2022). Hearing aids and cochlear implants in the prevention of cognitive decline and dementia—Breaking through the silence. JAMA Neurol. Published online Dec. 05, 2022. https://doi.org/10.1001/jamaneurol.2022.4155
- Dupuis, K., Marchuk, V., & Pichora-Fuller, M.K. (2016). Noise affects performance on the Montreal Cognitive Assessment. Canadian Journal on Aging, 35(3), 298-307. https://doi.org/10.1017/S0714980816000313
- Dupuis, K., Pichora-Fuller, M.K., Marchuk, V., Chasteen, A., Singh, G. & Smith, S.L. (2015). Effects of hearing and vision impairments on performance on the Montreal Cognitive Assessment. Aging, Neuropsychology, and Cognition, 22(4), 413-427. https://doi.org/10.1080/13825585.2014.968084
- Dupuis, K., Reed, M., Bachmann, F., Lemke, U., & Pichora-Fuller, M. K. (2019b). The circle of care for older adults with hearing loss and comorbidities: A case study of a geriatric audiology clinic. Journal of Speech, Language and Hearing Research, 62 (4S), 1203-1220. https://doi.org/10.1044/2018_JSLHR-H-ASCC7-18-0140
- Dupuis, K., Yusupov, I., Vandermorris, S., Murphy, K., Rewilak, D., Stokes, K., & Reed, M. (2019a). Considering age-related hearing loss in neuropsychological practice: Findings from a feasibility study. Canadian Journal on Aging, 38(2), 245-252. https://doi.org/10.1017/S0714980818000557
- Eberhard, J. M., Matthews, L. J., Vaden, K. I. Jr., Dubno, J. R., & Eckert, M. A. (2022). Probability distributions for associations between cognitive screening and pure-tone thresholds in older adults. Ear and Hearing. PAP Dec. 23, 2022. DOI: 10.1097/AUD.0000000000001313
- Ehrlich, J. R., Goldstein, J., Swenor, B. K., Whitson, H., Langa, K. M., & Veliz, P. (2022). Addition of vision impairment to a life-course model of potentially modifiable dementia risk factors in the US. JAMA Neurology, 79(6), 623-626. https://doi.org/10.1001/jamaneurol.2022.0723
- Gates, G. A., Cogg, J. L., Linn, R. T., Rees, T., Wolf, P. A., & D'Agostino, R. B. (1996). Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg, 122, 161-167. https://doi.org/10.1001/archotol.1996.01890140047010
- Gates, G. A., Anderson, M. L., McCurry, S. M., Feeney, M. P., & Larson, E. B. (2011) Central auditory dysfunction as a harbinger of Alzheimer’s dementia. Arch Otolaryngol Head Neck Surg, 137(4), 390-395. https://doi.org/10.1001/archoto.2011.28
- Giroud, N., Pichora-Fuller, M. K., Mick P., Wittich, W., Al-Yawer, F., Rehan, S., et al. (2021). Hearing loss is associated with gray matter differences in older adults at risk for and with Alzheimer’s disease. Aging Brain, 1, # 100018 https://doi.org/10.1016/j.nbas.2021.100018
- Glennon, E., Valtcheva, S., Zhu, A. Wadghiris, Y. Z, Svirsky, M. A., & Froemke, R. C. (2022). Locus coeruleus activity improves cochlear implant performance. Nature, Published online December 21, 2022. https://doi.org/10.1038/s41586-022-05554-8
- Hämäläinen, A., Pichora-Fuller, M. K., Wittich, W., Phillips, N. A., & Mick, P. (2021). Self-report measures of hearing and vision in older adults participating in the Canadian Longitudinal Study of Aging are explained by behavioral sensory measures, demographic, and social factors. Ear and Hearing, 42(4), 814–831. https://doi.org/10.1097/AUD.0000000000000992
- Hickson, L., Laplante-Lévesque, A., & Wong, L. (2013). Evidence-based practice in audiology: Rehabilitation options for adults with hearing impairment. American Journal of Audiology, 22(2), 329-331. https://doi.org/10.1044/1059-0889(2013/12-0085)
- Hoffmann, C. M., Nianogo, R. A., Yaffe, K., Rosenwohl-Mack, A., Carrasco, A., & Barnes, D. E. (2022). Importance of accounting for regional differences in modifiable risk factors for Alzheimer’s disease and related dementias: The case for tailored interventions. Journal of Alzheimer’s Disease, 89(2), 563–570. https://doi.org/10.3233/JAD-220278
- Huang, A. R., Jiang, K., Lin, F. R., Deal, J. A., & Reed, N. S. (2023). Hearing loss and dementia prevalence in older adults in the US. JAMA, 329(2), 171–173. https://doi.org/10.1001/jama.2022.20954
- Huang, A. R., Rebok, G. W., Swenor, B. K., Reed, N., Griswold, M., Zhu, Z., & Deal, J.A. (2022). Concurrent hearing and vision impairment and 8-year memory decline in community-dwelling older adults. Alzheimer’s & Dementia, 1-10. https://doi.org/10.1002/alz.12887
- Humes, L. E., & Dubno, J. R. (2021). A comparison of the perceived hearing difficulties of community and clinical samples of older adults. Journal of Speech, Language, and Hearing Research, 64(9), 3653–3667. https://doi.org/10.1044/2021_JSLHR-20-00728
- Humes, L. E., Kidd, G. R., & Lentz, J. J. (2013). Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Frontiers in Systems Neuroscience, 7, 55. https://doi.org/10.3389/fnsys.2013.00055
- Humes, L. E., Pichora-Fuller, M. K., & Hickson, L. (2020). Functional consequences of impaired hearing in older adults and implications for intervention (pp. 257-280). In K. Helfer, E. Bartlett, A. Popper, & R. R. Fay (Eds), Aging and hearing: Causes and consequences, Springer Handbook of Auditory Research 72. Springer Nature Switzerland AG. https://doi.org/10.1007/978-3-030-49367-7_11
- Ismail, Z. (2021). Early detection of Alzheimer disease – what do the new CCCDTD5 guidelines tell us? Webinar for the brainXchange: https://brainxchange.ca/Public/Events/Event-Archives/2021/Early-detection-of-Alzheimer-disease-%E2%80%93-what-do-the; https://vimeo.com/546121770
- Ismail, Z., Black, S., Camicioli, R., Chertkow, H., Herrmann, N., Laforce Jr., R., et al., (2020). Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimer’s Dement, 16, 1182-1195. https://doi.org/10.1002/alz.12105
- Lee, M., Whitsel, E., Avery, C., Hughes, T. M., Griswold, M. E., Sedaghat, S. et al. (2022). Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA network open, 5(7), e2219672. https://doi.org/10.1001/jamanetworkopen.2022.19672
- Leroi, I., Camacho, E., Chaghil-Boissier, N., Charalambous, A.P., Conelly, J., Constantinidou, F., et al. (2022). A Europe-wide randomized controlled trial of hearing and vision rehabilitation in dementia: Results from the SENSE-Cog trial. Alzheimer’s & Dementia, 18, e062722. https://doi-org.myaccess.library.utoronto.ca/10.1002/alz.062722
- Lindenberger, U., & Baltes, P. B. (1994). Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging, 9(3), 339–355. https://doi.org/10.1037/0882-7974.9.3.339
- Littlejohn, J., Bowen, M., Constantinidou, F., Dawes, P., Dickinson, C., Heyn, P., et al. (2022). International practice recommendations for the recognition and management of hearing and vision impairment in people with dementia. Gerontology, 68(2), 121–135. https://doi.org/10.1159/000515892
- Liu, C., Nagarajan, N., Assi, L., Jiang, K., Powell, D. S., Pedersen, E. et al. (2022). Assessment of hearing and vision impairment in cohort studies collecting cognitive data in older adults. Alzheimer’s & Dementia, 18, 2243– 2251. https://doi.org/10.1002/alz.12575
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D. et al. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673-2734. https://doi.org/10.1016/S0140-6736(17)31363-6
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S. et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413-446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., & Lawlor, B. A. (2018). Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia a systematic review and meta-analysis. JAMA Otolaryngology - Head and Neck Surgery, 144(2), 115–126. https://doi.org/10.1001/jamaoto.2017.2513
- Loughrey, D. G. (2022). Is age-related hearing loss a potentially modifiable risk factor for dementia? The Lancet Healthy Longevity, 3(12), e805-e806. https://doi.org/10.1016/S2666-7568(22)00252-5
- Lupien, S., Sindi, S., & Wan, N. (2012). When we test, do we stress? Guidelines for health professionals working with older adults. Centre for Studies on Human Stress, Montreal, Canada. https://www.humanstress.ca/Documents/pdf/KT/KT_document_EN.pdf
- Marinelli, J. P., Lohse, C. M., Fussell, W. L., Petersen, R. C., Reed, N. S., Machulda, M. M., et al. (2022). Association between hearing loss and development of dementia using formal behavioural audiometric testing within the Mayo Clinic Study of Aging (MCSA): a prospective population-based study. The Lancet Healthy Longevity, 3(12), e817-e824. https://www.thelancet.com/journals/lanhl/article/PIIS2666-7568(22)00241-0/fulltext
- Ma’u, E., Cullum, S., Cheung, G., Livingston, G., & Mukadam, N. (2021). Differences in the potential for dementia prevention between major ethnic groups within one country: A cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand. The Lancet regional health. Western Pacific, 13, 100191. https://doi.org/10.1016/j.lanwpc.2021.100191
- McMahon, C. M. (2021). Guest editorial: Hearing care for all—An opportunity to globally unite to address inequities in hearing health. Ear and Hearing, 42(3), 487-491. https://doi.org/10.1097/AUD.0000000000001047
- McMahon, C. M., Mosley, C. L., Pichora-Fuller, M. K., Davis, A. C., Baylor, C. R., Yorkston, K. M., & Tremblay, K. L. (2021). Older adults’ perceptions of current and future hearing healthcare services in Australia, England, US and Canada. Public Health Research & Practice, 31(5), e3152128. https://doi.org/10.17061/phrp3152128
- Mukadam, N., Marston, L., Lewis, G., & Livingston, G. (2022). Risk factors, ethnicity and dementia: A UK Biobank prospective cohort study of White, South Asian and Black participants. PloS one, 17(10), e0275309. https://doi.org/10.1371/journal.pone.0275309
- Mukadam, N., Sommerlad, A., Huntley, J., & Livingston, G. (2019). Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. The Lancet. Global health, 7(5), e596–e603. https://doi.org/10.1016/S2214-109X(19)30074-9
- Nalley, C. (2021). Practice benefits of integrating cognitive screening services. The Hearing Journal, 74(4), 26 - 28. DOI: 10.1097/01.HJ.0000743692.41362.c1 https://journals.lww.com/thehearingjournal/pages/articleviewer.aspx?year=2021&issue=04000&article=00001&type=Fulltext
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I. et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Nianogo, R. A., Rosenwohl-Mack, A., Yaffe, K., Carrasco, A., Hoffmann, C. M., & Barnes, D. E. (2022). Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurology, 79(6), 584–591. https://doi.org/10.1001/jamaneurol.2022.0976
- Owens, D., Davidson, K., Krist, A., Barry, M. J., Cabana, M., Caughey, A. B. et al. (2020). Screening for cognitive impairment in older adults: US Preventive Services Task Force Recommendation Statement Practice Guideline. JAMA, 323(8), 757-763. https://doi.org/10.1001/jama.2020.0435
- Panza, F., Lozupone, M., Sardone, R., Battista, P., Piccininni, M., Dibello, V., et al. (2018a). Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life. Therapeutic Advances in Chronic Disease, 1–17. https://doi.org/10.1177/2040622318811000
- Panza, F., Quaranta, N., & Logroscino, G. (2018b). Sensory changes and the hearing loss-cognition link: The cognitive ear. JAMA Otolaryngology - Head and Neck Surgery, 144(2), 127–128. https://doi.org/10.1001/jamaoto.2017.2514
- Panza, F., Solfrizzi, V., & Logroscino, G. (2015a). Age-related hearing impairment - a risk factor and frailty marker for dementia and AD. Nature Reviews Neurology, 11(3), 166–175. https://doi.org/10.1038/nrneurol.2015.12
- Panza, F., Solfrizzi, V., Seripa, D., Imbimbo, B. P., Capozzo, R., Quaranta, N., et al. (2015). Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Frontiers in Aging Neuroscience, 7, 113. https://doi.org/10.3389/fnagi.2015.00113
- Peelle, J. (2018). Listening effort: How the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear and Hearing, 39(2), 204-214. https://doi.org/10.1097/AUD.0000000000000494
- Petersen, R. C., & Yaffe, K. (2020). Issues and questions surrounding screening for cognitive impairment in older patients. JAMA, 323(8), 722-724. https://doi.org/10.1001/jama.2019.22527
- Phillips N. A. (2016). The implications of cognitive aging for listening and the Framework for Understanding Effortful Listening (FUEL). Ear and hearing, 37(Suppl 1), 44S–51S. https://doi.org/10.1097/AUD.0000000000000309
- Phillips, N., Isler, L., Kabir, R., Hämäläinen, A., Wittich, W., Pichora-Fuller, M. K., Mick, P. (2022). Hearing and visual acuity predict cognitive function in adults aged 45-85 years: Findings from the baseline wave of the Canadian Longitudinal Study on Aging (CLSA). Psychology and Aging. PAP November 11, 2022. https://doi.org/10.1037/pag0000716
- Pichora-Fuller, M.K. (2021). Auditory and cognitive processing (pp. 551-572). In J. Spitzer & J. Montano (Eds.), Adult audiologic rehabilitation: Advanced practices (3rd edition). Plural Publishing, San Diego, CA.
- Pichora-Fuller, M.K., Alain, C., & Schneider, B. (2017). Older adults at the cocktail party (pp. 227-259). In J. Middlebrooks, J. Simon, A. Popper, & R. R. Fay (Eds), The auditory system at the cocktail party, Springer Handbook of Auditory Research. Springer: Berlin. https://doi.org/10.1007/978-3-319-51662-2_9
- Pichora-Fuller, M.K., Dupuis, K., Reed, M., & Lemke-Kalis, U. (2013). Helping older people with cognitive decline communicate: Hearing aids as part of a broader rehabilitation approach. Seminars in Hearing, 34(4), 307-329. Special Issue on Cognition and Hearing Aids. http://dx.doi.org/10.1055/s-0033-1356643
- Pichora-Fuller, M.K., Mick, P.T., Reed, M. (2015). Hearing, cognition, and healthy aging: Social and public health implications of the links between age-related declines in hearing and cognition. Seminars in Hearing, 36, 122-139. Special Issue on Aging and Cognition. http://dx.doi.org/10.1055/s-0035-1555116
- Pichora-Fuller, M.K., & Singh, G. (2006). Effects of age on auditory and cognitive processing: Implications for hearing aid fitting and audiological rehabilitation. Trends in Amplification, 10, 29-59. https://doi.org/10.1177/108471380601000103
- Powell, D. S., Oh, E. S., Lin, F. R., & Deal, J. A. (2021). Hearing impairment and cognition in an aging world. Journal of the Association for Research in Otolaryngology: JARO, 22(4), 387–403. https://doi.org/10.1007/s10162-021-00799-y
- Presley, R. (2022). Cognivue technology is as effective as MoCA: What does this mean to audiologists, their practices, and patients?Continuing Education Course February 1, 2022.https://www.audiologyonline.com/interviews/innovations-summit-2022-cognivue-28135
- Reed, M., Freedman, M., Mark Fraser, A. E., Bromwich, M., Santiago, A. T., Gallucci, C. E., & Frank, A. (2022). Enhancing clinical visibility of hearing loss in cognitive decline. Journal of Alzheimer’s Disease, 86(1), 413–424. https://doi.org/10.3233/JAD-215377
- Rose, A.F., Gilbertson, A.F., Cottrell, C., & Tampi, R.R. (2021). Cognitive screening for adult psychiatric outpatients: Comparison of the Cognivue® to the Montreal Cognitive Assessment. World Journal of Psychiatry, 11(7), 265-270. https://doi.org/10.5498/wjp.v11.i7.265
- Sackett, D. L., Rosenberg, W. M. C., Gray, J. A. M., Haynes, R. B., & Richardson, W. S. (1996). Evidence based medicine: what it is and what it isn’t. The BMJ, 312, 71-72. https://doi.org/10.1136/bmj.312.7023.71
- Shilling-Scrivo, K., Mittelstadt, J., & Kanold, P.O. (2022). Decreased modulation of population correlations in auditory cortex is associated with decreased auditory detection performance in old mice. Journal of Neuroscience, 42(49), 9278-9292. https://doi.org/10.1523/JNEUROSCI.0955-22.2022
- Smid, J., Studart-Neto, A., César-Freitas, K. G., Dourado, M. C. N., Kochhann, R., Barbosa, B. J. A. P., et al. (2022). Subjective cognitive decline, mild cognitive impairment, and dementia - syndromic approach: Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dementia & Neuropsychologia, 16(3 Suppl 1), 1–24. https://doi.org/10.1590/1980-5764-dn-2022-s101en
- Thiyagarajan, J. A., Araujo de Carvalho, I., Peña-Rosas, J. P., Chadha, S., Mariotti, S. P., Dua, T., et al. (2019). Redesigning care for older people to preserve physical and mental capacity: WHO guidelines on community-level interventions in integrated care. PLoS Medicine, 16(10), e1002948. https://doi.org/10.1371/journal.pmed.1002948
- Vaden, K. I., Jr, Eckert, M. A., Matthews, L. J., Schmiedt, R. A., & Dubno, J. R. (2022). Metabolic and sensory components of age-related hearing loss. Journal of the Association for Research in Otolaryngology: JARO, 23(2), 253–272. https://doi.org/10.1007/s10162-021-00826-y
- Wallhagen, M. I., Strawbridge, W. J., & Tremblay, K. (2021). Leveraging the age friendly healthcare system initiative to achieve comprehensive, hearing healthcare across the spectrum of healthcare settings: an interprofessional perspective. International Journal of Audiology, 60(sup2), 80–85. https://doi.org/10.1080/14992027.2020.1853263
- Whitson, H. E., Cronin-Golomb, A., Cruickshanks, K. J., Gilmore, G. C., Owsley, C., Peelle, J. E., et al. (2018). American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: Sensory impairment and cognitive decline in older adults. Journal of the American Geriatrics Society, 66(11), 2052–2058. https://doi.org/10.1111/jgs.15506
- Wittich, W., Pichora-Fuller, M. K., Mick, P., & Phillips, N. A. (2022). Sensory health to support function and well-being in people living with dementia. In S. Gauthier, C. Webster, J. Morais, & P. Rosa-Neto (Eds). Alzheimer’s Disease International’s 2022 World Alzheimer Report “Post Diagnosis Support: Principles of Care”. https://www.alzint.org/resource/world-alzheimer-report-2022/
- Wong, L., & Hickson, L. (2012). Evidence-based practice in audiology: Evaluating interventions for children and adults with hearing impairment. Plural Publishing; San Diego, California.
- World Health Organization. (2019). Guidelines on risk reduction of cognitive decline and dementia. Geneva, Switzerland. https://www.who.int/publications/i/item/9789241550543
- World Health Organization. (2020). Healthy ageing and functional ability. Geneva, Switzerland. https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability
- World Health Organization. (2021a). Hearing screening: Considerations for implementation. Geneva, Switzerland. https://www.who.int/publications/i/item/9789240032767
- World Health Organization. (2017). Integrated care for older people (ICOPE): guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva, Switzerland. https://apps.who.int/iris/handle/10665/258981
- World Health Organization. (2001). International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland. https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health
- World Health Organization. (2022). World Rehabilitation Alliance. Geneva, Switzerland. https://www.who.int/initiatives/world-rehabilitation-alliance
- World Health Organization. (2021b). World Report on Hearing. Geneva, Switzerland. https://www.who.int/publications/i/item/world-report-on-hearing
- Yeo, B. S. Y., Song, H. J. J. M. D., Toh, E. M. S., Ng, L. S., Ho, C. S. H., Ho, R., et al. (2022). Association of hearing aids and cochlear implants with cognitive decline and dementia: A systematic review and meta-analysis. JAMA Neurology. Published online December 05, 2022. https://doi.org/10.1001/jamaneurol.2022.4427
- Yuan, J., Sun, Y., Sang, S., Pham, J. H., & Kong, W. J. (2018). The risk of cognitive impairment associated with hearing function in older adults: A pooled analysis of data from eleven studies. Scientific Reports, 8(1), 1–10. https://doi.org/10.1038/s41598-018-20496-w


