Active Transcutaneous Alternative for Patients with Conductive Hearing Loss


Oticon Medical’s new Sentio™ System provides a comfortable, effective listening experience to patients who would benefit from bone conduction treatment.
Oticon Medical Canada launched its first active transcutaneous bone anchored hearing system (BAHS), the Sentio System, on April 23, 2025, with an event in Quebec. The Sentio System is an active transcutaneous system that combines the Sentio Ti Implant, placed under the skin, and the Sentio 1 Mini sound processor, held over the implant by magnets.
The Sentio 1 Mini is the slimmest transcutaneous sound processor, providing remarkable sound quality and speech understanding. Sentio Ti Implant, the smallest transcutaneous implant¹, is designed for surgical flexibility and a straightforward procedure.
More than forty hearing care professionals attended the launch, including ENTs and audiologists. Invited speakers included James Tysome, MA, PhD, FRCS (ORL-HNS), Consultant ENT and Skull Base Surgeon of Cambridge University Hospitals (remotely) and wearer Wayne Lambert, who shared the story of how he became the first North American recipient of the Sentio System.
Wayne’s Story

Wayne Lambert, Oticon Medical Sentio wearer and his family
Wayne Lambert’s conductive hearing loss following treatment for cholesteatoma left him struggling to keep up with conversations. As a result, he stopped actively engaging with friends and family. His wife took notice and encouraged Wayne to seek treatment for his hearing difficulties.
"I did a lot of lip reading and I was in denial (about how) it affected me," Wayne explained. “My wife picked up on it real quick. I had to do something.”
Wayne learned about a new bone anchored hearing system for those experiencing similar conductive hearing losses. His surgeon felt the Sentio was an excellent option that would suit Wayne’s active lifestyle, which includes Crossfit and outdoor activities. “My (Sentio) device, it's magnetic, on my ear and I just pop it on. The only thing I ever have to check with it on maintenance is the battery. I can change my settings at any time to whatever my environment is.”
After the surgery and getting his processor, Wayne’s quality of life improved rapidly. He is grateful to once again fully engage with family and friends. “I love my device. It has improved my quality of life because now I can engage with my wife and my kids and my grandkids, as well as other people and not be isolated or feel isolated. I can be part of the conversation.”
Freedom of Choice
Oticon Medical prides itself on providing freedom of choice to wearers and their hearing healthcare providers. By offering the proven technology of the Ponto™ System in an active transcutaneous option, audiology professionals have more choices to offer their patients who could benefit from bone conduction treatment.
Counseling Session Guidance Online
Oticon Medical also offers an online tool to help hearing care professionals guide their patients through the journey to better hearing, including how to decide between a percutaneous or transcutaneous BAHS. This tool, BAHSjourney.com, can be used to present the entire journey or focus on a step or two, complete with handout materials and additional reading materials. Audiologists and ENTs can use this site while in session with a BAHS candidate to explain the treatment path and decide which bone conduction option would best suit the patient’s hearing needs, lifestyle, and preferences.
Cutting-edge Technology
The Sentio System delivers the proven benefits of the Ponto System—and more —in a transcutaneous option. Held in place by magnets, Sentio provides an alternative to percutaneous bone conduction options that require skin-protruding abutments. As such, it offers an additional solution for potential wearers and supports our commitment to freedom of choice.
Benefits to wearers
The Sentio 1 Mini processor is designed to fit seamlessly into a wearer’s daily routine. With its discreet size, others barely notice it. It stays securely in place, even in active situations. And with the industry’s widest bandwidth of 9.5 kHZ and OpenSound Navigator™, the Sentio 1 Mini gives wearers access to higher frequencies and a 360-degree sound experience. This is as close to natural hearing as possible, opening up everyday sounds, such as the tweeting of a bird.

It also delivers remarkable sound quality and speech understanding by effortlessly adapting to the environment. Wearers receive consistent and clear sounds and speech without audible feedback* thanks to OpenSound Optimizer.™ This technology will stop audible feedback before it even occurs.
Sentio 1 Mini is compatible with a wide range of wireless devices allowing wearers to stay in contact with loved ones, make calls, and watch television.
Benefits to hearing care professionals
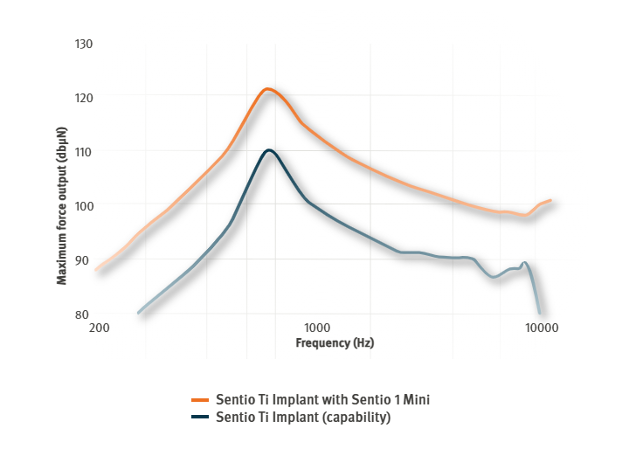
The Sentio Ti Implant is a SuperPowerful implant² developed to support progressive hearing loss without the need for an additional surgical procedure. It is designed and verified for higher maximum force output,** so that patients can rely on its ability to handle more powerful sound processors.
With its small size, the Sentio Ti Implant enables a straightforward surgical procedure that normally lasts less than an hour.³ The implant is designed to be flexible and easy to install with no or limited pre-operative planning.
Because Sound Matters
All our passion, knowledge, technology, and global resources are focused on supporting professionals and helping users overcome their hearing loss so they can live their lives to the fullest, now and in the future. Because we know the value of sound.
To learn more about the Sentio Systems features and benefits, please visit https://sentio.oticonmedical.com/ca.
To learn more about all of our products and becoming one of our valued clinical partners, please visit www.oticonmedical.com/ca.
References
- Sentio implant and sound processor physical features and comparison to other devices Doc-00123204
- 275144en Product Information Sentio 1 Mini
- Sentio study BC101 summary Doc-00123384
*For prescribed fittings, according to best practice and during normal use
** Maximum output vibratory force level on skull simulator, measured without any correction for placement²