Mysteries of the Hearing Brain
Biological Basis of Auditory Processing Deficits in Children
Some children with reading or language impairments show deficits in auditory processing. For example, children with developmental dyslexia perform more poorly than typically developing children when responding to speech stimuli that have been degraded by the presence of background noise or through the removal of temporal fine structure (vocoding).1 Nevertheless, the existence of an auditory processing deficit independent of language or cognitive impairment in these children is debated.2,3 Many of the tests used in the standard auditory processing battery require the ability to maintain attention or to retain a certain number of auditory items in memory. Alternatively, they may be affected by deficits in receptive or expressive language. Therefore, objective tests that do not require a behavioral response may be useful in diagnosing and managing these children.
Animal models provide compelling evidence of the existence of neurophysiological deficits that may contribute to impaired speech perception. For example, the quivering mouse carries a mutation in the βIV-spectrin gene that leads to ataxia and a type of central deafness. Specifically, the auditory brainstem response (ABR) of the quivering mouse is characterized by an absence of all waves above Wave I. The presence of Wave I indicates normal peripheral auditory function. Kopp-Scheinpflug and Tempel4 recorded neural responses to amplitude-modulated tones in quivering mice compared to wild type mice. They found that the timing (latencies) of single-neuron onset responses to these stimuli were jittered in the quivering mice rather than precisely aligned as they were in the wild type mice. These jittered neural responses result in delayed latencies and smaller amplitudes in population responses that can be detected at the scalp. Kopp-Scheinpflug and Tempel suggested that a similar mechanism may be responsible for the speech-evoked peak latency delays noted in the frequency-following responses (FFRs) of poor readers compared to FFR latencies of good readers.5 These and other studies provide evidence that an auditory neurophysiological deficit may contribute to some types of learning impairments.
The audiology field has made great strides in early identification of peripheral hearing loss through universal screening, with subsequently improved language outcomes for children with hearing loss.6 Language-based learning impairments are often not diagnosed until the child is in elementary school. If these deficits could be identified in infancy or early childhood, perhaps the outcomes in children with disorders such as specific language impairment or dyslexia could be improved as well. Infant cortical response may predict later language development,7, but the cortical auditory-evoked response has a wide range of normal variability, making it difficult to use this measure to identify abnormal responses in individual children. The FFR is less variable, and peak latency differences on milliseconds' order of fractions may be clinically significant.5
The Kraus lab at Northwestern University conducted a study in preschool-age children to determine whether the FFR could predict pre-reading skills assessed one year after the recording.8 They recorded responses to speech syllables presented in six-talker babble noise and combined measures of peak latencies, spectral energy, and response consistency to create a consonant-in-noise score. They found that this score predicted approximately 44% of the variance in a pre-reading score assessed one year later. Also, in a subset of children with a diagnosis of learning disability, they found that the consonant-in-noise score reliably classified the children into diagnostic categories 69% of the time, suggesting that this measure can be used to determine if an auditory processing deficit is contributing to the learning disability.
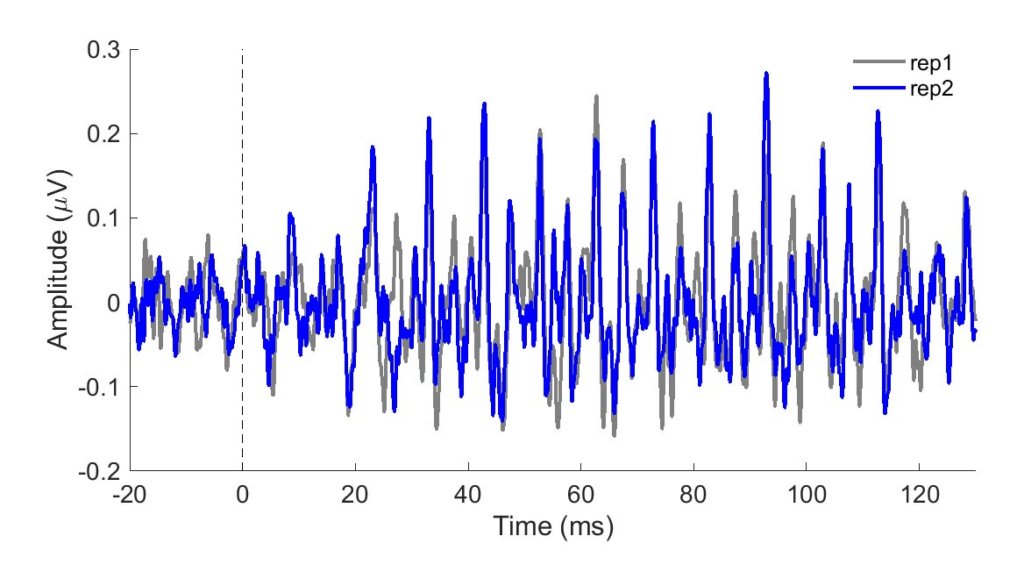
To be clinically useful in infants, one would need to ascertain that the neural recording can be reliably obtained. In Figure 1, two sets of responses recorded in a three-month-old are overlaid. One can see in this figure that the responses are highly replicable. The response "follows" the frequency of the stimulus in that the response peaks occur every 10 ms, a period corresponding to the 100-Hz fundamental frequency of the stimulus /ba/. The peaks are easily identified in these responses, but peak-picking may be more challenging in a noisier infant. For that reason, it may be useful to use an analysis that is not as subjective as peak picking. Figure 2 displays average phase locking from a study that assessed the development of phase-locking in infants.9
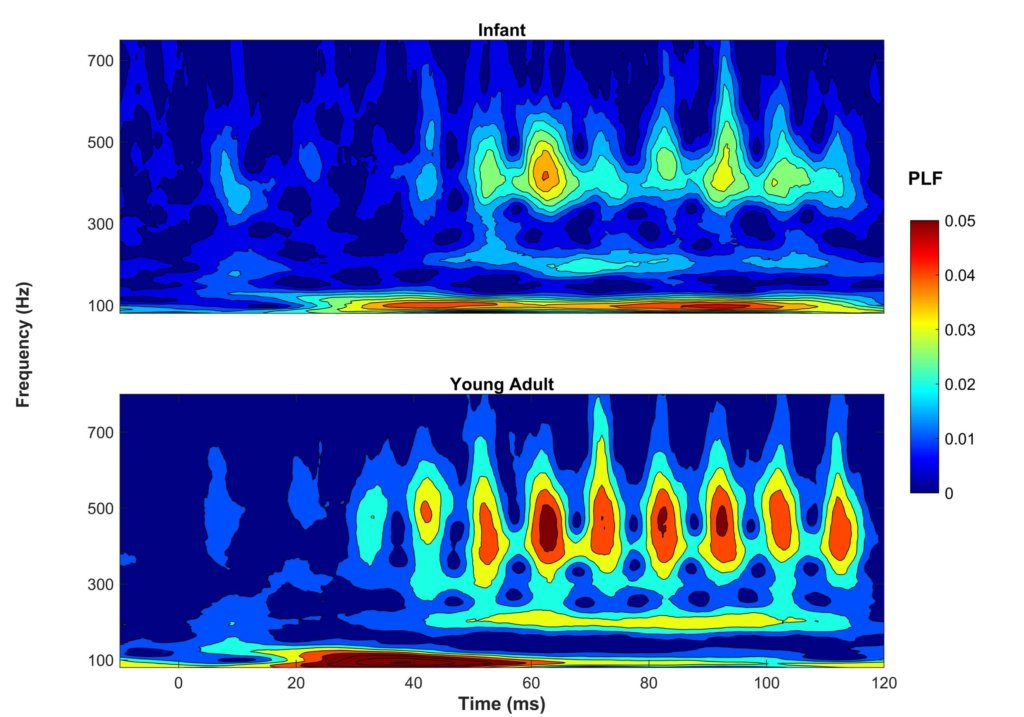
Phase-locking factor (PLF) was quantified by measuring the consistency of the response phase across sweeps. Hotter colors (red) indicate higher phase locking. One can see that in both infants (ages two to seven months) and young adults (ages 18–22), there is consistent phase locking to the 100-Hz fundamental frequency and harmonics. Ribas-Prats et al.10 recently published normative data from recordings to /da/ and /ga/ stimuli from 46 normal-hearing full-term neonates. These normative data include several measures in the time and frequency domains (e.g., signal-to-noise ratio, spectral amplitude). Researchers are currently investigating FFR variables that are most likely to predict language or reading impairments. I feel fortunate to be active in a field that continues to change and grow in exciting new areas. I expect that these investigations into the use of FFR will lead to significant changes in the ways we diagnose and manage infants and children with language-based learning impairments.
References
- Ziegler JC, et al. Speech-perception-in-noise deficits in dyslexia. Development Sci 2009;12:732–45.
- Iliadou V. and Kiese-Himmel C. Common misconceptions regarding pediatric auditory processing disorder. Frontiers Neurol 2018;8(732). 10.3389/fneur.2017.00732.
- Moore DR. Guest Editorial. Auditory Processing Disorder 2018;39(4):617–20. 10.1097/aud.0000000000000582.
- Kopp-Scheinpflug C. and Tempel BL. Decreased temporal precision of neuronal signaling as a candidate mechanism of auditory processing disorder. Hear Res 2015;330(Pt B):213–20. 10.1016/j.heares.2015.06.014.
- Banai K, et al. Reading and subcortical auditory function. Cerebral Cortex 2009;19(11):2699–707. 10.1093/cercor/bhp024.
- Yoshinaga‐Itano C. Early intervention after universal neonatal hearing screening: Impact on outcomes. Mental Retardation Development Disabil Res Rev 2003;9(4):252–66.
- Choudhury N and Benasich AA. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin Neurophysiol 2011;122(2):320–38.
- White-Schwoch T, et al. Auditory processing in noise: A preschool biomarker for literacy. PLoS Biol 2015;13(7):e1002196, 10.1371/journal.pbio.1002196.
- Van Dyke KB, et al. Development of phase locking and frequency representation in the infant frequency-following response. J Speech Language Hear Res: JSLHR 2017;60(9):2740–751. 10.1044/2017_jslhr-h-16-0263.
- Ribas-Prats T, et al. The frequency-following response (FFR) to speech stimuli: A normative dataset in healthy newborns. Hear Res 2019;371:28–39, https://doi.org/10.1016/j.heares.2018.11.001.

