Cochlear Excitotoxicity 101
This article is about cochlear excitotoxicity (as requested by our editor-in-chief). This topic has recently become of interest to us in audiology because of its involvement in inner hair-cell synaptic damage (synaptopathy) caused by acoustic overstimulation.1,2 However, long before the focus on “hidden hearing loss” we have known that cochlear excitotoxicity is responsible for the degeneration of spiral ganglion neurons after inner hair-cell loss, and it also underlies peripheral tinnitus generation immediately following acute cochlear injury.
In brief, excitotoxicity is the degeneration or death of neurons when over-activated by excessive amounts of neurotransmitters. Excitotoxicity is largely confined to neural systems that use the neurotransmitter glutamate, and given that glutamate is the predominant excitatory transmitter in the brain, it is not surprising that many brain pathologies result from or are exacerbated by excitotoxicity. For example, it contributes to traumatic brain injury, epilepsy, stroke, spinal cord injury, and certain neurodegenerative diseases. In most cases, there is some initial insult that damages a localized population of neurons. These degenerating cells release large quantities of glutamate neurotransmitter that causes excessive over-activation and ultimately the death of neurons in surrounding tissue. In this way, excitotoxicity is typically a secondary event, but often causing more extensive brain damage than the initial insult.
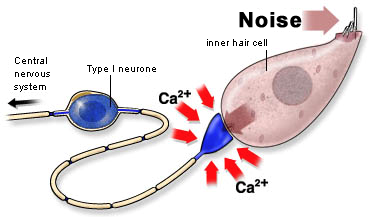
The basic mechanisms of glutamate excitotoxicity have been well studied. Essentially, glutamate neurotransmitter is released from a neuron (or a hair cell) and activates certain types of glutamate receptors at adjacent neurons' post-synaptic site. This initiates the opening of a limited number of ion channels in the cell membrane, resulting in the neuron's depolarization (activation). During this process, minimal amounts of sodium, potassium, and calcium ions leak across the cell membrane. However, when damaged cells release abnormally high or long-lasting glutamate levels, large numbers of membrane ion channels open and remain open in the connecting neurons, and ions flood into the cell. In particular, the high level of calcium ion influx is more than homeostatic mechanisms of the cell can handle, and this most often leads to apoptosis (i.e., cell death).3
In the cochlea, connections between inner hair cells and spiral ganglion neurons are mediated by the neurotransmitter glutamate; therefore, these synapses can cause and be damaged by excitotoxicity. Indeed, cochlear exposure to loud sounds, direct mechanical injury to the inner ear, certain ototoxic drugs, and some genetic mutations can result in cochlear inner hair cell damage, and this, in turn, can cause excitotoxic degeneration of spiral ganglion cells.
Cochlear excitotoxicity can occur with various degrees of severity. Exposure to intense acoustic signals or direct trauma to the cochlea can damage inner hair cells, and then through excitotoxic effects, damage or kill spiral ganglion cells. The result can be a permanent hearing loss. During the period when spiral ganglion cells (a.k.a. cochlear afferent neurons) are over-excited and rapidly firing action potentials, there is a resulting subjective percept of tinnitus. This form of tinnitus lasts only as long as the excitotoxic events, which in time will reduce as the affected neurons die completely or perhaps recover. You may be interested in a rather unique experimental study by my research team at SickKids on the time-course of excitotoxicity induced by injury to the cochlea.4
With acoustic overstimulation at levels that are too low to injure hair cells permanently, they can nevertheless damage the synapses of inner hair cells. This type of inner hair cell synaptopathy can cause permanent excitotoxic degeneration of spiral ganglion cells and the survival of inner hair cells. This is the basis for our current understanding of the cause of "hidden hearing loss.”1,2
I will write more on this in the future but will finish this article with a mention of a future avenue of research relating to cochlear excitotoxicity. Given the importance of this pathological mechanism in contributing to hearing dysfunction (including tinnitus), interventions may become available to prevent excitotoxicity. For example, a very recent publication5 has described pharmacological treatments that might block or deactivate the cell membrane channels that allow massive calcium influx and cell death in excitotoxicity. This and other agents could offer protection against noise-induced cochlear injury (and the associated hearing loss).
References
1. Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci 2009;29:14077–85.
2. Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 2011;12:605–16.
3. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010;47:122–29.
4. Negandhi J, Harrison AL, Allemang C, Harrison RV. The time course of cochlear injury discharge (excitotoxicity) determined by ABR monitoring of the contralateral cochlear events. Hear Res 2014;26;315:34–39.
5. Hu N, Rutherford MA, Green SH. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. PNAS 2020;117(7):3828–38.