How Close Are We To Cochlear Hair Cell Regeneration In Humans?
From the Labs to the Clinics
Renowned auditory researcher Dr. Robert Harrison brings us up to date on information and research from the Labs. Appropriately titled “From the Labs to the Clinics”, Bob is involved in laboratory and applied/clinical research, including evoked potential and otoacoustic emission studies and behavioural studies of speech and language development in children with cochlear implants. For a little insight into Bob’s interests outside the lab and the clinic, we invite you to climb aboard Bob’s Garden Railway.
Many decades ago, there was much excitement in the audiology community about the possibility of hearing restoration by somehow promoting cochlear hair cell regeneration. Many possible mechanisms for achieving this goal were discussed, including introducing stem cells that could develop into hair cells, or inducing a conversion of supporting cells into hair cells with gene therapy or other pharmacologic treatments. Optimists and dreamers predicted the replacement of hearing aids and cochlear implants by “regenerative medicine” methods. The future of clinical audiology (in its present state) and the hearing instruments industry could be forever changed.
I thought it was time for our “labs to clinic” column to issue an update. I am largely depending on a recent (2023) review in the journal Pharmaceutics titled: Future Pharmacotherapy for Sensorineural Hearing Loss by Protection and Regeneration of Auditory Hair Cells1. Many years have passed, and an incredible amount of research time and energy (and money) has been spent, but we do not appear to be anywhere near the end of the tunnel. There was much optimism about rapid progress after discovering important cellular pathway components such as ATOH1 (required for differentiation of auditory hair cells in the developing ear), and Notch pathway inhibitors (the notch signaling pathway regulates cell proliferation, differentiation, and cell death). However, after many experiments in lower vertebrates and immature or in vitro ears (cell cultures), there has been much progress in the lab to understand the biology of hair cell development, but very limited knowledge translation to the clinic. There has been little progression to clinical (human) trials.
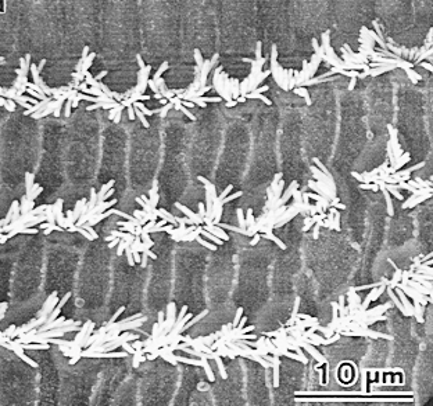
Image courtesy of Dr. R.V. Harrison, Auditory Science Laboratory, SickKids Toronto.
Certainly, human trials have demonstrated the safety and efficacy of delivery of genes or drugs into the inner ear with adenovirus vectors or intratympanic injection. For example, a 2023 clinical trial of ATOH1 gene therapy reports that intracochlear drug delivery with an adenovirus is safe but that “no meaningful increase in hearing was identified.”2 In a very recent clinical trial where Notch inhibitors were administered by intratympanic injection, again the only the safety of the drug application was demonstrated, with no evidence of hearing restoration reported yet.3
To end on a positive note, there has been one clinical trial in which some patients with SNHL showed “clinically meaningful improvements” in word recognition scores.4 This was a unique trial to see if supporting cells could be transformed into “pluripotent” stem cells and then into hair cells, using a combination of VPA (valproic acid, an anti-cancer agent that play an important role in cell regulation, inducing cell death (apoptosis) and cell cycle arrest) and FX-322 (a potent activator of the Wnt signaling pathway that regulates crucial aspects of cell development). This clinical trial, reported in 2021 by McClean and colleagues, was a high-quality study carried out by an international team of well-qualified experts. The results were encouraging, but I am presently unaware of any follow-up studies.
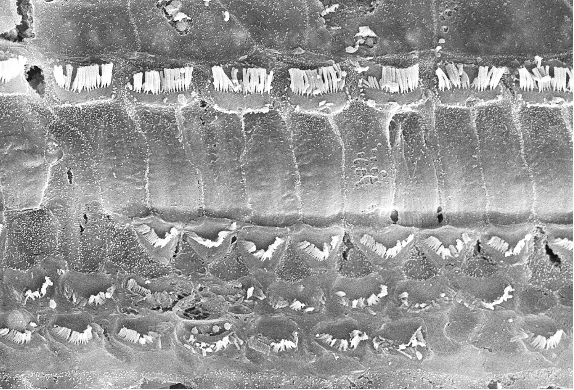
Image courtesy of Dr. R.V. Harrison, Auditory Science Laboratory, SickKids Toronto.
This short report for Canadian Audiologist is a rather cursory overview of a very large body of scientific enquiry. In summary, we can note that in recent times, many clinical trials have been initiated to test various hair cell regeneration strategies in subjects with stable SNHL. Drug administration has largely been achieved with local intratympanic and intracochlear methods, and there are useful findings regarding the safety and tolerability of these application routes. However, other than the McLean et al. study, significant hearing recovery has not been reported in clinical trials for hair cell regeneration,4 which reported limited beneficial effects. Furthermore, in non-human (adult mammal) studies no experiments have reported significant hair cell regeneration or hearing restoration. So, audiologists and the hearing prosthesis industry can breathe easily. There is still much work and time required to achieve the goal of an easy fix for SNHL.
References
- Matsunaga M, Nakagawa T. Future Pharmacotherapy for Sensorineural Hearing Loss by Protection and Regeneration of Auditory Hair Cells. Pharmaceutics. 2023; 15(3):777. https://doi.org/10.3390/pharmaceutics15030777
- 023 Clinical Trial of ATOH1 Gene Therapy. Results summary:[https://www.novctrd.com/ctrdweb/patientsummary/patientsummaries?patientSummaryId=680
- Results of clinical trial of γ-secretase inhibition of Notch signaling
https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-004544-10/results
- McLean WJ, Hinton AS, Herby JTJ, Salt AN, Hartsock JJ, Wilson S, Lucchino DL, Lenarz T, Warnecke A, Prenzler N, Schmitt H, King S, Jackson LE, Rosenbloom J, Atiee G, Bear M, Runge CL, Gifford RH, Rauch SD, Lee DJ, Langer R, Karp JM, Loose C, LeBel C. Improved Speech Intelligibility in Subjects With Stable Sensorineural Hearing Loss Following Intratympanic Dosing of FX-322 in a Phase 1b Study. Otol Neurotol. 2021 Aug 1;42(7):e849-e857. doi: 10.1097/MAO.0000000000003120.

