The Risks of Explaining Hearing Loss as a Potentially Modifiable Risk Factor for Dementia –Summer 2024 Update on New Global and Canadian Population Attributable Fractions (PAFs)

This update on publications concerning population-attributable fractions (PAFs) for potentially modifiable risk factors for dementia focuses on hearing loss and new analyses using global and Canadian data. A mini-tutorial on PAF statistics is provided to help audiologists understand the PAF findings, how to interpret them, and their implications for policy and practice.
Mini-tutorial on the PAF statistic
General: The PAF (also known as population-attributable risk, or PAR) is an estimate of the proportion of a disease (or adverse condition) caused by a risk factor in a certain population (Mansournia & Altman, 2018). The word “attributable” implies a causal link between the risk factor and the outcome. The PAF is the maximum proportion of disease incidence that would disappear from a population if the risk factor were eliminated. The PAF is calculated as a function of two variables: (1) the prevalence of a risk factor and (2) its relative risk (RR) with respect to the disease in question. Prevalence is the proportion of all people in the population with the risk factor (in contrast to prevalence, incidence refers only to new cases with the risk factor). The RR is the risk of developing the disease among people with the risk factor compared to those without the risk factor. The RR depends on population characteristics (e.g., sex, age) and how the risk factor is defined.
PAFs for dementia risk: A comment about the PAFs for dementia risk (Kivipelto et al., 2024) stresses that
“It is important to acknowledge that the calculation of the population attributable fraction relies on assumptions, such as that risk factors are truly causal and can be eliminated. Also, these statistics are, by necessity, highly aggregated and might not fully account for synergistic or antagonistic interactions among risk factors or the fact that these factors can contribute differently to various types of dementia, such as Alzheimer’s disease and vascular dementia, which can co-occur.”
These caveats are imperative to understanding the meaning of PAFs at the population level. For example, it is improbable that hearing loss could ever be eliminated from the population, even with better primary prevention strategies. Furthermore, the interpretation of the PAF rests on causal assumptions. Causal mechanisms linking hearing loss to dementia are plausible but unproven. Furthermore, given that both hearing loss and dementia are strongly associated with aging, it is plausible that one or more common underlying age-related mechanisms may account for at least some of the observed associations between hearing loss and dementia (i.e., there could be a common cause that accounts for both hearing loss and dementia without hearing loss itself directly causing dementia). Treatments for hearing loss do not eliminate it, and normal hearing is not restored, even if treatments mitigate the effects of hearing loss. Crucially, there is still no convincing evidence based on RCT studies that treating hearing loss reduces the risk of dementia. In addition to the issue of there being no proven causal mechanism to account for the association between hearing loss and dementia, there are other reasons why the oft-cited PAF of hearing loss for all-cause dementia must be qualified. At best, the PAF is a rough marker of the relative importance of hearing loss relative to other risk factors for dementia, but it may not be equivalent or even valid for all populations or sub-groups in a population. Publications in 2024 also show that the PAF may change over time. Indeed, if progress is made in overcoming a risk factor, then the PAF should shrink. Clinicians must recognize that the PAF has limitations as a population indicator of risk. It is even more important for clinicians to recognize that the PAF cannot be used to estimate individual risk. The PAF may inform decisions about population-level public health programs, but not to quantify the risk status of individual patients.
Overview of 2023-2024 publications on PAFs for dementia risk
Hearing loss was reported to be the greatest potentially modifiable risk factor for dementia (PAF of 8.2%) in previously published reports by the Lancet Commission on Dementia Prevention, Intervention and Care (Livingston et al., 2017, 2020). In July 2023, the first results of the Aging and Cognitive Health Evaluation in Elders (ACHIEVE) randomized control trial (RCT) were released at the Alzheimer’s Association International Conference (AAIC) and in a paper published in the Lancet (Lin et al., 2023). Recall that ACHIEVE is the first RCT to investigate if comprehensive audiologic rehabilitation (not simply hearing aid use) could actually reduce the rate of decline in cognitive performance (see Pichora-Fuller, 2023a). The ACHIEVE results released in July 2023 showed no significant overall effect of audiologic rehabilitation on the rate of cognitive decline. There was slower cognitive decline attributed to audiologic rehabilitation in a specific high-risk sub-group that began the study with poorer cognitive performance and other risk factors for dementia (smoked more, less education, more often lived alone, and more likely to have diabetes and hypertension). However, there were not enough cases to study dementia as an outcome over the three years of the ACHIEVE study. Therefore, despite the relatively high PAF of 8.2% for hearing loss as a potentially modifiable risk factor for dementia that had been reported, the first results of the ACHIEVE RCT did not provide evidence that treatment for hearing loss could actually reduce the incidence of dementia. At the July 2024 AAIC, a year after the release of the first results of the ACHIEVE RCT, an update to the 2020 report of the Lancet Commission on Dementia Prevention, Intervention, and Care was presented and published in the Lancet with revised PAF statistics used to estimate the potentially modifiable risk factors for dementia (Livingston et al., 2024). Using global data, the PAF for hearing loss decreased from 8.2% (2020) to 7% (2024). Below are highlights of some of the updates on these global PAFs, some recent findings from studies on Canadian PAFs, and some explanations of what Canadian audiologists might need to understand about PAFs for the purposes of counselling older people living with hearing loss.
Comparisons of 2020 and 2024 PAFs for dementia risk factors
All 14 risk factors for dementia: From the 2020 report (Livingston et al., 2020) to the 2024 report (Livingston et al., 2024), the PAFs changed for 7 of the 12 risk factors included in both reports. Hearing loss had the highest PAF (8.2%) in 2020, but in 2024 the PAF for hearing loss tied (7%) with the PAF for high LDL cholesterol, a newly added risk factor. In 2024, vision loss was also added as a new risk factor, with a PAF of 2%. Comparisons from 2020 to 2024 were as follows for the PAFs of the other 11 previously identified modifiable risk factors for dementia: increases were reported for three factors: social isolation (4 to 5%), air pollution (2 to 3%) and diabetes (1 to 2%); decreases were reported for three factors: low education (7 to 5%), smoking (5 to 2%) and depression (4 to 3%); no change was reported for five factors: traumatic brain injury (3%), hypertension (2%), physical inactivity (2%), obesity (1%) and excessive alcohol consumption (1%). In 2020, the combined PAF for 12 risk factors was 40% and it rose to 45% in 2024 for 14 risk factors (LDL cholesterol and vision loss were the two new risk factors). The 2024 report also considered a number of other factors (e.g., sleep, diet, infections and systemic inflammation, bipolar disorder, psychotic disorders, anxiety, menopause and hormone replacement therapy, multimorbidity and frailty) for which there was insufficient evidence to warrant adding them as new risk factors; however, it seems likely that future reports may include these or other factors as new research emerges. The 2024 report also considers the evidence regarding screening and diagnosis for dementia, including possible benefits as well as possible harms, and concludes that
“The balance of evidence and ethical principles finds that people should have access to timely and accurate diagnosis with appropriate interventions when they are seeking help, but the evidence does not justify screening the whole population for dementia.”
(pp. 32).
Hearing loss as a risk factor for dementia: In 2020, hearing loss was defined as a better-ear four-frequency pure-tone threshold average > 25 dB HL and the prevalence in older adults was estimated at 31.7% globally; prevalence was estimated to be higher (59%) in 2024. In 2020, the RR values for hearing loss were determined by meta-analysis (i.e., calculating weighted averages) using data from 3 studies: two from the United States and one from the United Kingdom (which included only men). Five new meta-analyses were used in the 2024 calculations. The RR for hearing loss was 1.9 in 2020, meaning that the risk of developing all-cause dementia for people with hearing loss was 1.9 times the risk for those who did not have hearing loss, even after multivariable adjustment for relevant confounders in statistical models; the RR was lower (1.4) in 2024. As with prevalence, the RR may vary according to population characteristics (e.g., severity of hearing loss within the group of people with hearing loss). Furthermore, some risk factors may cluster together in individuals due to unifying, underlying causes (i.e., “latent factors”); for example, a latent socioeconomic factor may explain clustering of hypertension, smoking, diabetes, and low education in some individuals in a specific population. Adjustment for such communalities was performed so that PAF values would better reflect the proportion of disease uniquely attributed to each risk factor. In 2020, communality for hearing loss was 45.6%, and in 2024 it increased to 60.9%.
The calculations for hearing loss included all people without considering whether or not they used hearing aids. It was noted that evidence for the benefits of hearing aid use for dementia risk is increasing, including evidence from a systematic review and meta-analysis (Yeo et al., 2023) based on eight studies of which two studies found significant benefits (but see Denham et al., 2023; Pichora-Fuller, 2023c) and the results for the high-risk sub-group in the ACHIEVE RCT (Lin et al., 2023). Although there is ample evidence that audiologic rehabilitation benefits communication and there are likely interactions between these benefits and other risk factors for dementia (e.g., physical activity, social isolation), there is still insufficient evidence that hearing aid use alters risk for dementia. The conclusion about hearing loss as a potentially modifiable risk factor for dementia is best situated in the context of the overall conclusion that
“Key individual interventions are preventing and treating hearing loss, treating vision loss and depression, cognitive stimulation throughout life, decreasing smoking, reducing and treating vascular risk factors (i.e., cholesterol, diabetes, obesity, and blood pressure), reducing head injury, and maintaining and encouraging physical activity. Policy changes can improve education (in quality as well as years of education) and reduce smoking, alcohol use, the risk of TBI, air pollution, and salt and sugar in food, thus targeting obesity, hypertension, and diabetes. Structural changes can help to increase exercise and reduce social isolation. Notably, interventions or lifestyle changes at any stage of life can alter the risk of dementia.”
(pp. 42-43, Livingston et al., 2024).
Hearing loss is only one of a growing set of risk factors for dementia. The communalities among risk factors and variations in the data used to estimate prevalence and RR limit the extent to which the PAF values for a single risk factor can be isolated, and there are also limits on how PAF values can be generalized.
Generalizability
Globally: The 2024 Lancet Commission report (Livingston et al., 2024) conveys a very strong message that the global PAF statistics must be qualified depending on social determinants of health (e.g., differences due to socio-economic status within countries and between high- and low-income countries). Crucially, the calculated PAFs may not be valid for populations with different prevalence and RR values (if population-specific RR values are known). A rapidly growing literature shows that dementia risk varies over time and across countries; for example, there have been declines in the prevalence and incidence of dementia in England, Spain, Sweden, France, USA, but an increase in Japan (Avan & Hachinski, 2024). Furthermore, a review of 27 studies (Mukadam et al., 2024) indicates that differences in the prevalence of risk factors for dementia could affect PAFs; in general, PAFs for less education and/or smoking have declined while PAFs for obesity, hypertension and diabetes have increased, with most data coming from high-income countries. For 15 of these 27 papers, there was sufficient data to calculate changes over time in PAFs for at least two factors by using the same RR values used in the 2020 Lancet Commission report (Livingston et al., 2020). Of these 15 papers, only two papers yielded data on changes in PAFs for hearing loss. Over roughly 30 years (1975 to 2005), the PAF for hearing loss changed from 2.5 to 2.8% in a Swedish study (Sacuiu et al., 2020). Over about 20 years (1989 to 2011), the PAF for hearing difficulties changed from 5.8 to 6.8% in a British study (Matthews et al., 2016). Notably, a comment (Smith & Deal, 2024) on a systematic review and meta-analysis paper concerning PAFs (Stephan et al., 2024) urged researchers “to consider person (effect of co-occurring risk factors), place (reporting PAF estimates specific to the regions in which interventions are likely to be deployed), and time (a life course approach) when calculating and reporting PAF”. This is also an important message for clinicians who are interpreting research reporting PAFs.
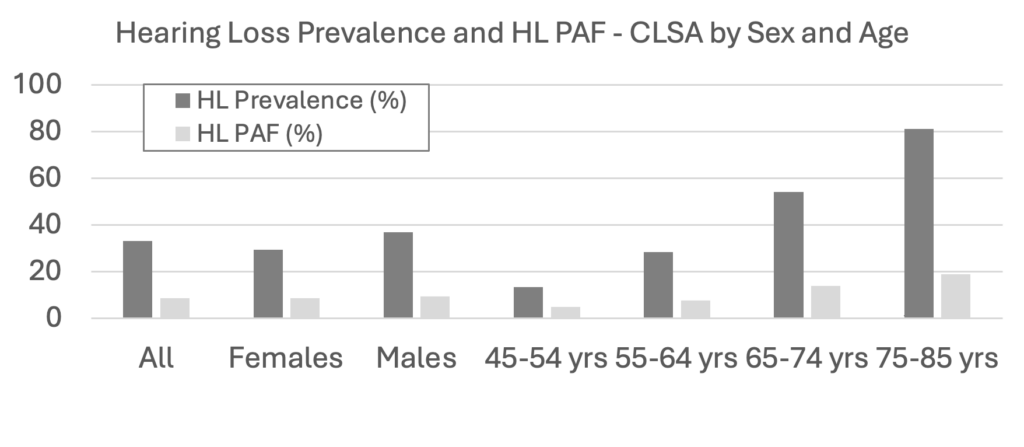
Canada: In particular, it is important for Canadian audiologists to be aware of how these qualifying issues have emerged in papers using prevalence data from over 30,000 participants in the Canadian Longitudinal Study on Aging (CLSA; Raina et al., 2009; https://www.clsa-elcv.ca/). One Canadian paper (Son et al., 2024) calculated PAFs for 11 of the 12 risk factors (air pollution was excluded) using the same RR values as had been used in the 2020 Lancet Commission report (Livingston et al., 2020) and sleep disturbances was added as an additional risk factor. Prevalence for the risk factors was based on baseline data from the CLSA (but Canada-specific RRs were not available). Canadian data were compared to similar data available for eight other countries (Australia, Brazil, China, Denmark, India, Latin America, New Zealand, USA). The PAF for the combined proportion of dementia risk factors explained by the 11 risk factors that were common across countries was highest in Latin America (55.9%) and lowest in Denmark (35.2%) which was similar to Australia (36%), with the PAF for Canada being 49.2%. Striking differences in PAFs were found when CLSA data analyses were conducted by sex and age. For hearing loss, prevalence was 33.2%, and the PAF was 8.7%. However, as shown in Figure 1, PAF depends on prevalence; for example, the prevalence of hearing loss is greater for males than females and increases with age. This pattern illustrates how the characteristics of the population may affect the PAF estimates for hearing loss in Canada.
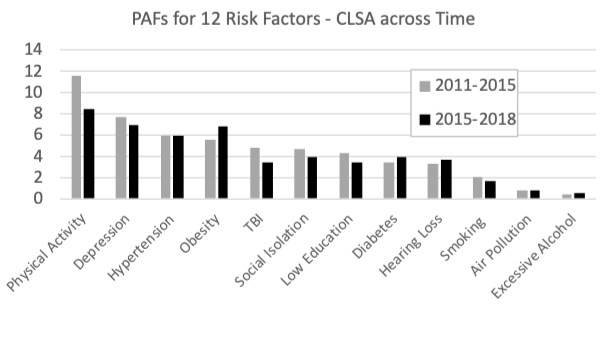
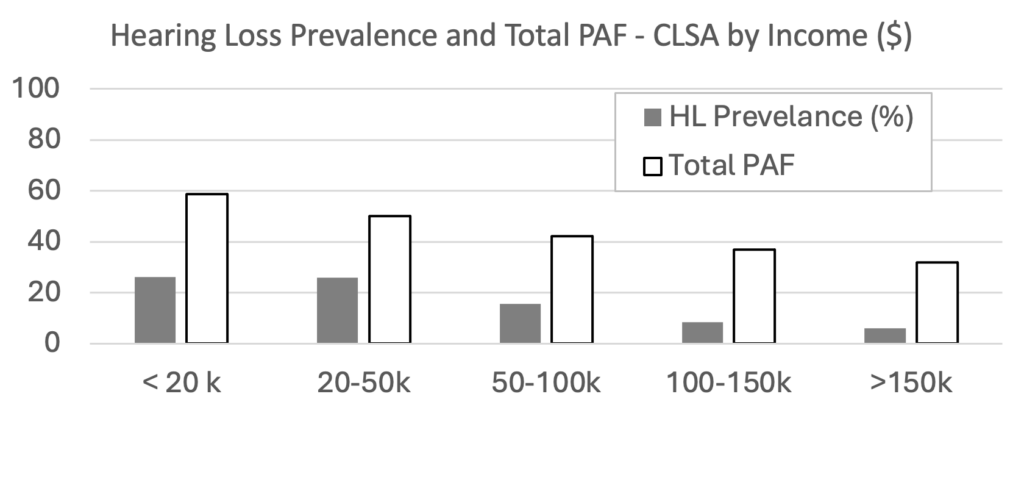
Another Canadian paper (Dolatshahi et al., 2024) compared CLSA data from two time points, baseline (2011-2015) and the first followup (2015-2018). As shown in Figure 2, over time, the PAF was unchanged for air pollution, decreased for 7 of the 12 risk factors (physical activity, depression, hypertension, TBI, social isolation, low education, smoking), and increased for 4 risk factors (obesity, diabetes, hearing loss, and excessive alcohol consumption). This pattern is similar to the general pattern observed across high-income countries (Mukadam et al., 2024). At baseline the combined PAF for all 12 risk factors was 43.4% and at followup it was 40.1%. Importantly, however, as shown in Figure 3, the combined PAF depended on income, with the risk factors explaining more dementia risk for those with lower incomes than for those with higher incomes. At baseline the prevalence of hearing loss was 15.7% and the PAF for hearing loss was 3.3% (ranked 9th highest of the same 12 risk factors as had been reported in the 2020 Lancet Commission report (Livingston et al., 2020)). At followup, the prevalence of hearing loss was 16.2% and the PAF for hearing loss was 3.7% (ranked 7th of 12). The discrepancies between the two Canadian papers are likely explained by the inclusion of slightly different sets of risks factors and different applications of weighting factors for the population; however, the patterns of PAFs are similar, with physical activity having the highest PAF and hearing loss having a relatively low PAF when CLSA data is used. It is also noteworthy that, as shown in Figure 3, the combined PAF for all risk factors and the prevalence hearing loss varies with income, with the total combined PAF and the prevalence of hearing loss being greater for those with lower incomes than for those with higher incomes. It follows that the contribution of hearing loss to the combined PAF for dementia risk would depend on socio-economic factors related to various social determinants of health.
Discussion
There are several take-away messages for Canadian audiologists:
- The PAF statistic can be used at the population-level, but it does not quantify an individual’s risk.
- At the population-level, caution is needed in interpreting global or Canadian PAF statistics because these are expected to change over time and they depend on sub-population characteristics (e.g., sex, age, income) that may be related to social determinants of health.
- The PAF for hearing loss has relatively high communality with other risk factors, so it is best to consider it one of many potentially modifiable risk factors. In Canada, as in other high-income countries, people living with hearing loss who are concerned about reducing risk for dementia, including people who have already benefited from audiologic rehabilitation, may find it worthwhile to learn about the importance of non-audiological factors such as improving management of treatable comorbid health conditions (depression, hypertension, obesity, diabetes) and making lifestyle changes (e.g., more physical activity, more social engagement, not smoking, not consuming excessive amounts of alcohol) that could benefit many aspects of health (e.g., cardiovascular, brain health), including hearing health (WHO, 2021, 2023). In particular, it seems reasonable that better hearing (and vision) could help people to become more engaged in physical and social activities that could yield multiple health benefits. Audiologists could consider hearing solutions that might improve communication accessibility in situations conducive to increasing physical and social activities.
- Future research in hearing healthcare should investigate how best to integrate hearing healthcare into inter-professional integrated primary care for older people (WHO, 2017; Pichora-Fuller, 2023b). Indeed, in a comment on the 2024 Lancet Commission report (Kivipelto, et al., 2024), it was noted that
“Increasing evidence shows that multidomain interventions, targeting several risk factors simultaneously, are feasible and effective for people with increased risk for dementia. These interventions rely on the application of the principle that one size does not fit all; therefore, to be effective and feasible, interventions should be tailored to risk profiles and adapted to geographically, culturally, and economically diverse populations globally. Several multidomain trials of risk reduction are in progress worldwide. …. Some of these trials are applying the precision prevention approach, optimizing the multidomain intervention for specific risk profiles.”
References
- Avan, A., & Hachinski, V. (2024). Increasing risks of dementia and brain health concerns. Comment, the Lancet Public Health, 9(7), e414-e415. DOI:https://doi.org/10.1016/S2468-2667(24)00123-3
- Denham, M. W., Weitzman, R. E., & Golub, J. S. (2023). Hearing aids and cochlear implants in the prevention of cognitive decline and dementia—Breaking through the silence. JAMA Neurol., 80(2), 127-128. https://doi.org/10.1001/jamaneurol.2022.4155
- Dolatshahi, Y., Mayhew, A., O’Connell, M.E., Liu-Ambrose, T., Taler, V., Smith, E. E., et al. (2024). Prevalence and population attributable fractions of potentially modifiable risk factors for dementia in Canada: A cross-sectional analysis of the Canadian Longitudinal Study on Aging. Can J Public Health. Published online July 24, 2024. https://doi.org/10.17269/s41997-024-00920-7
- Kivipelto, M., Mangialasche, F., & Anstey, K. J. (2024). Pivotal points in the science of dementia risk reduction. The Lancet, Comment published online July 31, 2024https://doi.org/10.1016/S0140-6736(24)01546-0
- Lin, F. R., Pike, J. R., Albert, M. S., Arnold, M., Burgard, S., Chisolm, T., Couper, D., et al. (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. The Lancet (British Edition). https://doi.org/10.1016/S0140-6736(23)01406-X
- Livingston, G., Huntley, J., Liu, K. Y., Costafreda, S. G., Selbæk, G, Alladi, S., et al. (2024). Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. The Lancet, published online July 31, 2024 https://doi.org/10.1016/S0140-6736(24)01296-0
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S. et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413-446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D. et al. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673-2734. https://doi.org/10.1016/S0140-6736(17)31363-6
- Mansournia, M. A., & Altman, D. G. (2018). Population attributable fraction. BMJ, 360, k757. doi: 10.1136/bmj.k757. https://www.bmj.com/content/360/bmj.k757
- Matthews, F. E., Stephan, B. C., Robinson, L., Jagger, C., Barnes, L. E., Arthur, A., & Brayne, C. (2016). Cognitive Function and Ageing Studies (CFAS) Collaboration. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun, 7, 11398. doi: 10.1038/ncomms11398.
- Mukadam, N., Wolters, F. J., Walsh, S., Wallace, L., Brayne, C., Matthews, F. E., et al. (2024). Changes in prevalence and incidence of dementia and risk factors for dementia: an analysis from cohort studies. The Lancet Public Health, 9(7), e443-e460. DOI: https://doi.org/10.1016/S2468-2667(24)00120-8
- Pichora-Fuller, M. K. (2023a). Findings from the ACHIEVE RCT: Does hearing care modify dementia risk? Canadian Audiologist, 10(5). https://canadianaudiologist.ca/findings-from-the-achieve-rct/
- Pichora-Fuller, M. K. (2023b). Hearing care in integrated person-centered care for older adults: Can audiologic rehabilitation help in meeting the key challenge areas for aging well in Canada? Canadian Audiologist, 10(6). https://canadianaudiologist.ca/12507-2/
- Pichora-Fuller, M. K. (2023c). Is hearing loss in older adults predictive of later development of dementia and does hearing care modify dementia risk? Canadian Audiologist, 10(1). https://canadianaudiologist.ca/issue/volume-10-issue-1-2023/is-hearing-loss-in-older-adults-predictive-of-later-development-of-dementia-and-does-hearing-care-modify-dementia-risk/
- Raina, P. S., Wolfson, C., Kirkland, S. A., Griffith, L. E., Oremus, M., Patterson, C. et al. (2009). The Canadian longitudinal study on aging (CLSA). Can J Aging, 28(3), 221–9. doi:https://doi.org/10.1017/S0714980809990055
- Sacuiu, S., Gustafson, D., Sjögren, M., Guo, X., Ostling, S., Johansson, B., & Skoog, I. (2010). Secular changes in cognitive predictors of dementia and mortality in 70-year-olds. Neurology, 75(9), 779-85. doi: 10.1212/WNL.0b013e3181f0737c.
- Smith, J. R., & Deal, J. A. (2024). The need for a more holistic approach to dementia prevention. Lancet Healthy Longevity, 5(6), e382-e383. https://doi.org/10.1016/S2666-7568(24)00071-0
- Son, S., Speechley, M., Zou, G.Y., Kivipelto, M., Mangialasche, F., Feldman, H. H., et al. (2024). Potentially modifiable dementia risk factors in Canada: An analysis of Canadian Longitudinal Study on Aging with a multi-country comparison. J Prev Alzheimers Dis. Published online June 2024. https://doi.org/10.14283/jpad.2024.105
- Stephan, B. C. M., Cochrane, L., Kafadar, A. H., Brain, J., Burton, E., Myers, B., et al. (2024). Population attributable fractions of modifiable risk factors for dementia: A systematic review and meta-analyses. Lancet Healthy Longevity, 5, e406-21. https://doi.org/10.1016/S2666-7568(24)00061-8
- Yeo, B. S. Y., Song, H. J. J. M. D., Toh, E. M. S., Ng, L. S., Ho, C. S. H., Ho, R., et al. (2023). Association of hearing aids and cochlear implants with cognitive decline and dementia: A systematic review and meta-analysis. JAMA Neurology, 80(2), 1340141. https://doi.org/10.1001/jamaneurol.2022.4427
- World Health Organization. (2023). Dementia fact sheet. https://www.who.int/news-room/fact-sheets/detail/dementia
- World Health Organization. (2017). Integrated care for older people (ICOPE): guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva, Switzerland. https://apps.who.int/iris/handle/10665/258981
- World Health Organization. (2021). World Report on Hearing. Geneva, Switzerland. https://www.who.int/publications/i/item/world-report-on-hearing

