From the Labs to the Clinics
From the Labs to the Clinics
Renowned auditory researcher Dr. Robert Harrison brings us up to date on information and research from the Labs. Appropriately titled “From the Labs to the Clinics”, Bob is involved in laboratory and applied/clinical research, including evoked potential and otoacoustic emission studies and behavioural studies of speech and language development in children with cochlear implants. For a little insight into Bob’s interests outside the lab and the clinic, we invite you to climb aboard Bob’s Garden Railway.
Gene Therapy for Hearing Loss; An Update on the Evidence
A few weeks back our editor-in-chief Marshall Chasin sent me a media headline proclaiming a quick fix for hearing loss, and he invited my comments.
This “breakthrough” looked exciting, implying that a quick needle jab was all that was required to reverse hearing loss, and the comment “researcher says this kind of treatment for deafness is “just the beginning”. I was intrigued, but after spending half a century in scientific research on deafness, I knew it was, to say the least, a bit of an exaggeration! The sensational headlines were from THE INDEPENDENT, a UK tabloid newspaper reporting for the lay audience on serious research on gene therapy for children with hearing loss due to otoferlin gene mutations.
Last year I gave more accurate details on the latest research progress in this area: (Gene Therapy for Children with Deafness: We Are Almost There, At Least for Single Gene Mutations. Canadian Audiologist Vol 11, Issue 3). I decided that a follow-up report might be useful.
Before that, I want to comment on the contrast between the newspaper headline story and accurate scientific reporting. Both are based on the outcome of clinical trials of gene therapy, but written for different audiences and conveying different messages. This news media article highlights the stark difference between published scientific data, and the common press. It should remind us to be careful of where we look to find accurate facts or evidence. We are entering an era when fake news and alternative facts are pervasive and often persuasive.
So, back to science. The research publication (in The Lancet) that made news last year was titled “AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial”.1 AAV stands for the adeno-associated virus that is used to transport and transfect OTOF gene into cochlear haircells. A diagrammatic overview of the route of the genetic material to the target site is shown in the figure below.
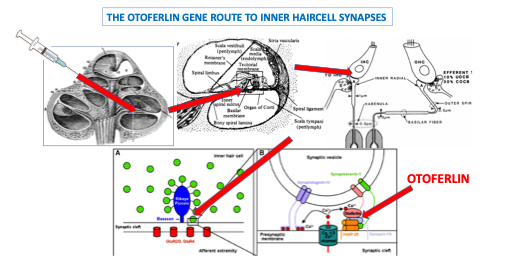
The paper reported preliminary findings in a clinical trial of gene therapy for otoferlin gene-related auditory neuropathy. To remind you, this is a promising advancement in the treatment of genetic hearing loss, particularly OTOF-related deafness. Mutations in OTOF prevent the function of inner hair cell synapses, causing congenital auditory neuropathy. A single gene mutation causes this particular hearing loss. Relatively few types of genetic hearing loss are cause by single gene mutations, so this treatment can be considered as the most straightforward type of gene therapy (sometimes described as the low-hanging fruit).
The publication was ground-breaking in that it showed proof of concept and procedural safety, but it was too soon to get definitive proof of efficacy. There were some indications of hearing improvements, but the age and number of subjects precluded getting statistically significant results.
Since their 2024 paper, this team has recently published a follow up commentary in The Lancet: “OTOF-related gene therapy: a new way but a long road ahead”.2 As the title implies, they report that there is much to be learned about gene therapy clinical trials, and whilst the basic concept of replacing a bad gene with a good one sounds straightforward, this therapy still has a “long road ahead”. The authors assert that OTOF gene therapy still faces substantial technical and clinical challenges, noting for example that the large size of the OTOF gene requires innovative delivery strategies. They warn that immune responses to AAV-OTOF could lead to health risks such as inflammation and cochlear damage.
Of interest, the authors discuss the age of the patients and the importance of early treatment, but report unpublished findings that a 23-year-old showed “promising results”. This suggests that adults with OTOF-related deafness might also benefit from gene therapy, but this remains speculative.
Because of the potential of gene therapy in many clinical areas, including hearing healthcare, there have been several recent commentaries discussing the scientific concepts and challenges of clinical trials. I mention a few below, not in detail, but readers might be interested in the overviews and current opinions in the field of OTOF gene therapy.
A commentary in the Journal of the American Medical Association (JAMA) is titled “Gene Therapy for Hearing Loss-Will the Price Be Right?”3. The authors draw attention to the high cost of gene therapy, and the need for “innovative financing models” to ensure accessibility to this treatment.
An editorial in the Annals of Medicine and Surgerytitled: “Breaking the silence: gene therapy offers hope for OTOF-mediated hearing loss”4 provides a concise overview of the issues to date.
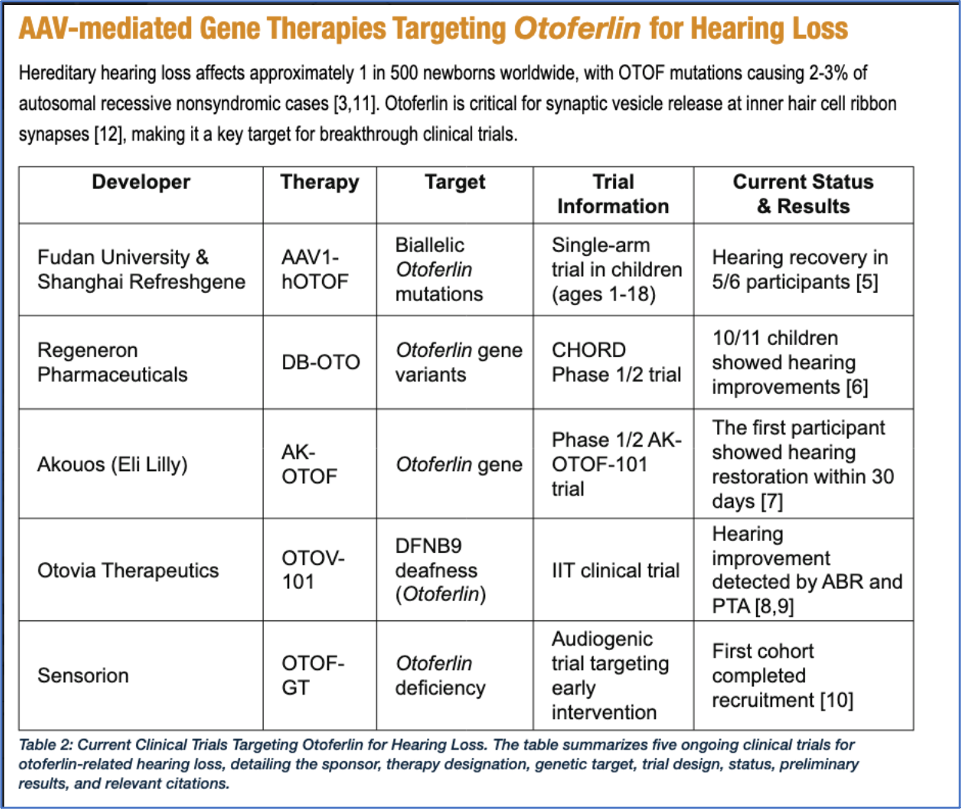
Lawrence Lustig has written a useful review in Current Opinion in Otolaryngology & Head and Neck Surgery: “Cochlear gene therapy for otoferlin-related hearing loss”5. He notes that there are several international teams involved in human gene therapy trials for otoferlin-associated hearing loss. For example: the Eye and ENT Hospital Fudan University (China), Lilly-Akouos (USA), Otovia (China), Regeneron (USA), and Sensorion (France). This review summarizes early work that led to these efforts and highlights early published data on clinical outcomes. Published outcomes are currently limited because some trials are still in progress and data collection is incomplete. So far the treatments all appear safe with limited adverse effects associated with the therapies. All groups report varying degrees of hearing improvement following cochlear gene therapy, and recent data suggest that improvement is not limited only to young children but also to some adolescents.
The current focus on otoferlin gene therapy is largely because this is a single gene mutation, and the target structures of the inner ear, i.e., inner hair cell synapses, are almost fully intact and “ready to go” when the good gene is inserted. However, this genetic etiology is rare compared with the connexin gene mutations that are responsible for a large proportion of congenital deafness. A report in Molecular Therapy by Lukas Landegger, titled “Novel AAV-based GJB2 gene therapy restores hearing function”6 reports on (the limited) progress in GJB2 gene therapy.
Finally, for this update I highly recommend a review article by E. Khadir, E. and L. Byelyayeva: “Gene Therapy for Hearing Loss: A Translational Development Roadmap”7. This waspublished in May 2025, online in World Pharma Today. (See the URL in references.) It is an excellent overview of the state of the field and outlines a translational roadmap from preclinical development to clinical implementation. I have reproduced a table from this article that summarizes the teams currently involved in otoferlin gene therapy and preliminary results where available.
References
- Lv, J., Wang, H., Cheng, X., Chen, Y., Wang, D., Zhang, L., Cao, Q., Tang, H., Hu, S., Gao, K. and Xun, M., 2024. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. The Lancet, 403(10441), pp.2317-2325.
- Jieyu Qj, Lei Xu, Fan-Gang Zeng, Renjie Chai.: OTOF-related gene therapy: a new way but a long road ahead. The Lancet, Volume 405, Issue 10481 p777-779 DOI: 10.1016/S0140-6736(25)00248-X
- Miller LE, Adunka OF, Rathi VK. Gene Therapy for Hearing Loss—Will the Price Be Right? JAMA Otolaryngol Head Neck Surg. 2025;151(4):291–292. doi:10.1001/jamaoto.2024.5189
- Hussain, Syed Ahmed Shahzaeem; Ali, Mohammad Haris; Imtiaz, Muhammad Hassan; Al Hasibuzzaman. Breaking the silence: gene therapy offers hope for OTOF-mediated hearing loss, editorial. Annals of Medicine & Surgery 86(9): 4950-4951, September 2024. DOI: 10.1097/MS9.0000000000002360
- Lustig, L., Cochlear gene therapy for otoferlin-related hearing loss. Current Opinion in Otolaryngology & Head and Neck Surgery, pp.10-1097. DOI: 10.1097/MOO.0000000000001070
- Landegger, Lukas D. Novel AAV-based GJB2 gene therapy restores hearing function. Molecular Therapy, Volume 33, Issue 7, 2957-2958.
- Khadir, E. and Byelyayeva, L., Gene Therapy for Hearing Loss: A Translational Development Roadmap. Published online in World Pharma Today. https://www.cilcare.com/wp-content/uploads/2025/05/GeneTherapy-WPTarticle-print.pdf