Is there a Role for Evoked Potentials in the Hearing Aid Fitting?
As a practicing audiologist, I was often frustrated when I could not find adequate solutions for people who were struggling to adapt to hearing aids. Although technology has improved dramatically since I started working as an audiologist 35 years ago, some patients continue to experience problems with sound quality, performance in noise, etc. I found that I could not predict who was going to have problems with hearing aids based on the information obtained from the audiologic evaluation. These observations led me to pursue research to investigate the underlying factors contributing to poor speech-in-noise performance in older adults, and to investigate solutions addressing these factors with devices (hearing aids or cochlear implants) and/or training. My hypothesis was that the lack of hearing aid acceptance may be due in part to age- and hearing-related changes in the central processing of sound in the auditory nerve, brainstem, or cortex that affect the neural representation of the speech signal.
I use electrophysiology to study effects of aging, hearing loss, amplification, and training on neural processing of speech. My primary focus has been on the frequency following response (FFR), a measure arising primarily from the brainstem/midbrain regions that closely resembles the stimulus in timing and frequency characteristics. In a series of studies, I found that the neural response degrades with age. This degradation can be seen as a reduction in amplitude (Figure 1), delayed latencies, decreased phase locking, etc.1–3
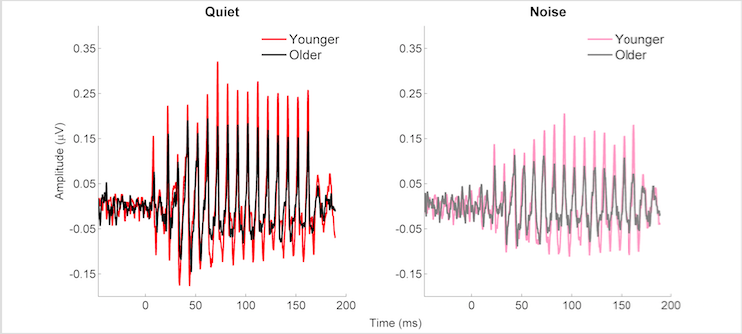
Figure 1. Frequency following response waveforms to the speech syllable /da/ obtained in younger (red/pink) and older (black/gray) adults. These responses were recorded in quiet (red/black) and noise (competing talker at -6 dB SNR) (pink/gray). In both quiet and noise conditions, the amplitude of the older adults’ waveforms is significantly reduced compared to that of the younger adults. Adapted from Presacco et al., 2016.3
The reduction in amplitude shown in Figure 1 may result from temporal jitter associated with synaptopathy,4 a decrease in auditory nerve fibres5 or other age-related factors in the central auditory system. Temporal jitter or disrupted timing leads to a reduction in neural synchrony; neural synchrony is important for accurate transmission of the speech signal and is especially important in noise. An extreme example of this timing disruption would be Auditory Neuropathy Spectrum Disorder (ANSD). Some older adults may have a mild form of this disorder, which would explain their difficulty with hearing aids. Hearing aids improve audibility but do not restore a disrupted neural timing mechanism.
Despite the potential limitations of hearing aids for restoring disrupted neural timing, the use of electrophysiology (EEG) may have diagnostic or management value for improving the fitting. There has been recent interest in the use of cortical auditory evoked potentials (CAEPs) and the FFR to objectively assess speech audibility in babies and other individuals who are difficult-to-test. Currently, real-ear measurement is the standard of care for verifying hearing aid function in infants and adults. Although this measure is important for ensuring that hearing aids are providing appropriate sound levels, it does not provide information about what the individual is actually perceiving. The HEARLab® Cortical Auditory Evoked Potential Analyzer uses a statistical algorithm to objectively determine if a response is detectable in the auditory cortex and is now commercially available. This statistical algorithm was used to verify an increase in response detectability for various speech sounds in infants with sensorineural hearing loss.6
CAEPs are affected by sleep and may therefore be problematic when testing young infants or those who are unable to cooperate for EEG testing without some type of sedation. The extremely brief tone pips and clicks used in auditory brainstem response testing would be ineffective for evaluating hearing aids with processing times in milliseconds. However, the FFR can be obtained with longer speech stimuli and is relatively unaffected by sleep or sedation; it may therefore be a viable alternative to CAEP testing. Easwar and colleagues conducted two studies to investigate the feasibility of using the FFR (referred to in these studies as the envelope following response, EFR) to objectively assess hearing aid benefit. In both studies, they used a speech-like stimulus token /susaʃi/, which was designed to elicit neural responses to eight different frequency regions. They used a statistical algorithm to determine whether or not a response was present. The first study was conducted in young adults with normal hearing and found that the EFR increased in amplitude with an increase in stimulus level for all frequency ranges.7 An increase in bandwidth also increased the number of response detections. Their follow-up study, conducted in older adults with hearing loss, confirmed that the EFR was sensitive to changes in level and bandwidth with amplification and can be accomplished in clinically feasible test times (~20 minutes).8 Importantly, they found that EFR amplitude and detectability correlated with speech discrimination scores and sound quality ratings.
These studies demonstrate the feasibility of using CAEPs/FFRs to demonstrate increased audibility with hearing aids in individuals who are unable to provide a behavioral response. It may also be beneficial to develop a measure that can compare the effects of different hearing aid algorithms or settings on the fidelity of neural speech representation. A preliminary study showed that FFR parameters changed with different hearing aid settings in an older individual with hearing loss (Figure 2).9 Therefore, differences in algorithms might be systematically assessed in different populations to determine which settings produce the best neural response and behavioral performance. For example, studies have shown that slow compression attack and release times, which preserve the temporal envelope, may be better for older individuals with poorer cognitive ability than fast attack and release times.10,11 But some hearing aid platforms recommend assessing the activity level of an individual’s lifestyle to determine which algorithm works best. Activity level and cognitive function may not be equivalent. Furthermore, there is little evidence for setting compression speeds for young people with hearing loss who might also benefit from a preserved temporal envelope. We are currently assessing the effects of varying hearing aid compression parameters on neural response fidelity in younger and older adults with hearing loss. We hope that this information will be useful in future development of hearing aid algorithms and for helping audiologists and hearing instrument dispensers to make evidence-based decisions.
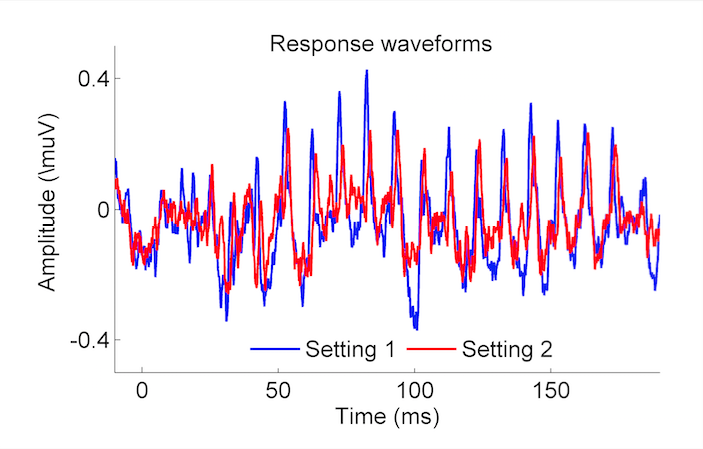
Figure 2. FFR waveforms recorded in an older individual with hearing loss to the speech syllable /da/. This individual was wearing hearing aids programmed with Setting 1 (Blue) or Setting 2 (Red), and an increase in response amplitude was noted for setting 2. Anderson and Kraus, 2013.9
In summary, EEG testing has the potential to improving the listener’s experience with hearing aids. Future studies may provide guidance for setting specific hearing aid parameters and may lead to clinical assessments for predicting success with hearing aids in individual listeners.
References
- Anderson S, Parbery-Clark A, White-Schwoch T, et al. Aging affects neural precision of speech encoding. J Neurosci 2012; 32:14156–164.
- Presacco A, Jenkins K, Lieberman R, et al. Effects of aging on the encoding of dynamic and static components of speech. Ear Hear 2015;36(6):e352–63. doi: 10.1097/AUD.0000000000000193.
- Presacco A, Simon JZ, Anderson S. Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol 2016; n.00372.2016. doi: 10.1152/jn.00372.2016. [Epub ahead of print]
- Sergeyenko Y, Lall K, Liberman MC, et al. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J Neurosci 2013;33:13686–694.
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 1996;76:2799–803.
- Chang H-W, Dillon H, Carter L., et al. The relationship between cortical auditory evoked potential (CAEP) detection and estimated audibility in infants with sensorineural hearing loss. Intl J Audiol 2012;51:663–70.
- Easwar V, Purcell DW, Aiken SJ, et al. Effect of stimulus level and bandwidth on speech-evoked envelope following responses in adults with normal hearing. Ear Hear 2015;36:619–34.
- Easwar V, Purcell DW, Aiken SJ, et al. Evaluation of speech-evoked envelope following responses as an objective aided outcome measure: Effect of stimulus level, bandwidth, and amplification in adults with hearing loss. Ear Hear 2015;36:635–52.
- Anderson S, Kraus N. The potential role of the cABR in assessment and management of hearing impairment. Int J Otolaryngol 2013; doi: 10.1155/2013/604729. Epub 2013 Jan 30.
- Lunner T, Sundewall-Thoren E. Interactions between cognition, compression, and listening conditions: Effects on speech-in-noise performance in a two-channel hearing aid. J Am Acad Audiol 2007; 18.
- Souza PE, Arehart KH, Shen J, et al. Working memory and intelligibility of hearing-aid processed speech. Front Psychol 2015;6:526.

