Towards Better Understanding of SP/AP Area Ratio of ECochG
Electrocochleaography (ECochG) is an almost 90-year-old auditory evoked potential test that is used to assess the inner ear functions. It is well known as a diagnostic test of endolymphatic hydrops. ECochG is also used to determine the site of lesions in cases of Auditory Neuropathy Spectrum Disorders and categorize them accordingly as presynaptic or postsynaptic. Nowadays, ECochG is gaining popularity as test for noise-induced cochlear synaptopathy or what is called hearing loss. ECochG responses are believed to originate from four cellular sources, the outer hair cells (OHCs), the inner hair cells the dendritic potentials and spikes of auditory nerve fibers (ANFs).
ECochG response is composed of three components cochlear microphonics (CMs), Summating Potentials (SP) and Action Potentials (AP). CMs are alternating current potentials which originate from the hair cells, especially OHCs. SP is a direct current response generated by the hair cells of the organ of corti and a reflection of the displacement –time pattern of the cochlear partition. AP is the summed response of numerous, at times thousands, of ANFs firing synchronously. That is why it is called Compound AP.
ECochG can be recorded using different types of electrodes; Tiptrode or gold foil electrodes are used to record ECochG from external auditory canal, Tymptrode is used to record ECochG from tympanic membrane and transtympanic electrode which is used to record ECochG from cochlear promontory through an opening on the tympanic membrane. There is no doubt that the use of transtymapnic electrode would give the best responses, yet being an invasive technique, it can only be conducted by a trained physician. This limits its clinical use.
The most important parameter in the analysis of the ECochG response is measuring the amplitude of SP and AP to derive the SP/AP amplitude ratio. It is known that an elevated SP relative to AP is a common finding in cases of Endolyphatic Hydrops (ELH) /Meniere’s disease (MD). The incidence of an elevated SP/AP amplitude ratio is only 60% in MD patients. This low sensitivity of SP/AP amplitude ratio motivated researchers to find ways to improve its sensitivity for diagnosis of MD. Consideration of the response duration and the amplitude of the ECochG components were the bases for measuring the area of SP/AP complex.
Studies have shown that SP-AP complex is widened with trailing edge of the AP in MD, which was explained by Morrison et al. as prolonged after-ringing of the CMs caused by ELH. The widening of SP/AP complex was found to be more obvious with the use of alternating polarity click stimuli because of the latency difference between the condensation and rarefaction components associated with ELH.1 Another explanation of the prolonged SP-AP complex in patients with MD are the changes in the speed of the travelling wave, as an endolymph-loaded basilar membrane has restricted upward and downward movement.
There are different methods to calculate the SP/AP amplitude and area ratio. Ferraro and his collaborators used the following formula to measure SP/AP amplitude ratio.2–4
The SP/AP ratio used by Ferraro and his collaborators is different than the mathematical formula calculating the area under the curve and it was based on theoretical speculations about prolonged SP-AP complex in MD. They have reported increased sensitivity of ECochG in MD using SP/AP area ratio up to 92% (Figure 1 and Figure 2).
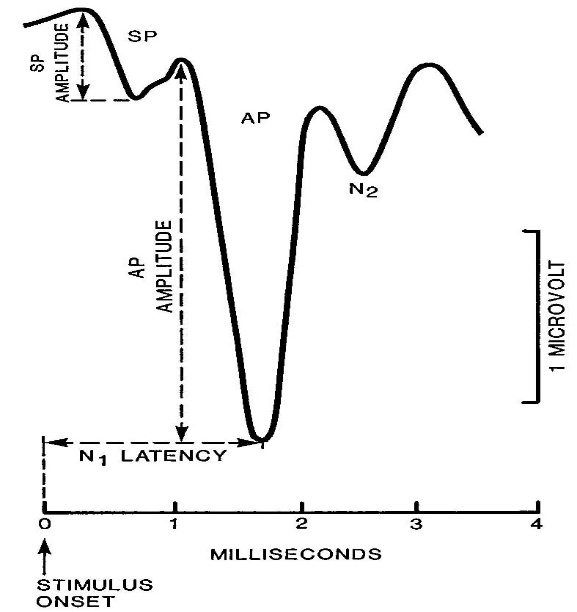
Figure 1. SP/AP amplitude ratio. A cut point of 0.41 was considered abnormal by the Ferraro group
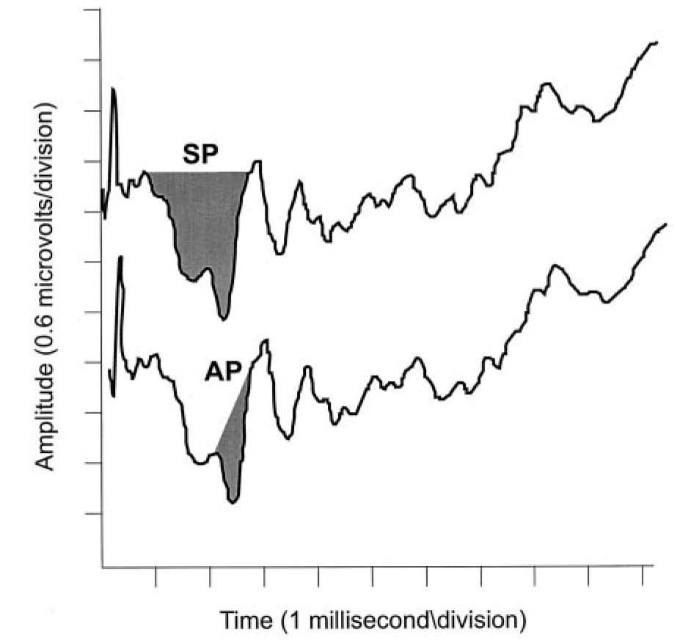
Figure 2. SP/AP area ratio. A cut point of 1.37 was considered abnormal by the Ferraro group.
There is concern that their method was developed on theoretical bases and it might be vulnerable to normally variant morphology of SP-AP waveform, and consequently it is vulnerable to increased false positive results or reduced specificity. Actually, there is some controversy in the literature regarding the value of SP/AP area ratio. For example, Baba et al. reported that (SP/AP) area ratio may not necessarily have higher sensitivity in the diagnosis of endolymphatic hydrops of MD.
After examination of the SP/AP ratio in the published literature and in different Evoked Potential systems, surprisingly we have found significant variations in methods used for calculation of the SP/AP ratio (Figure 3).
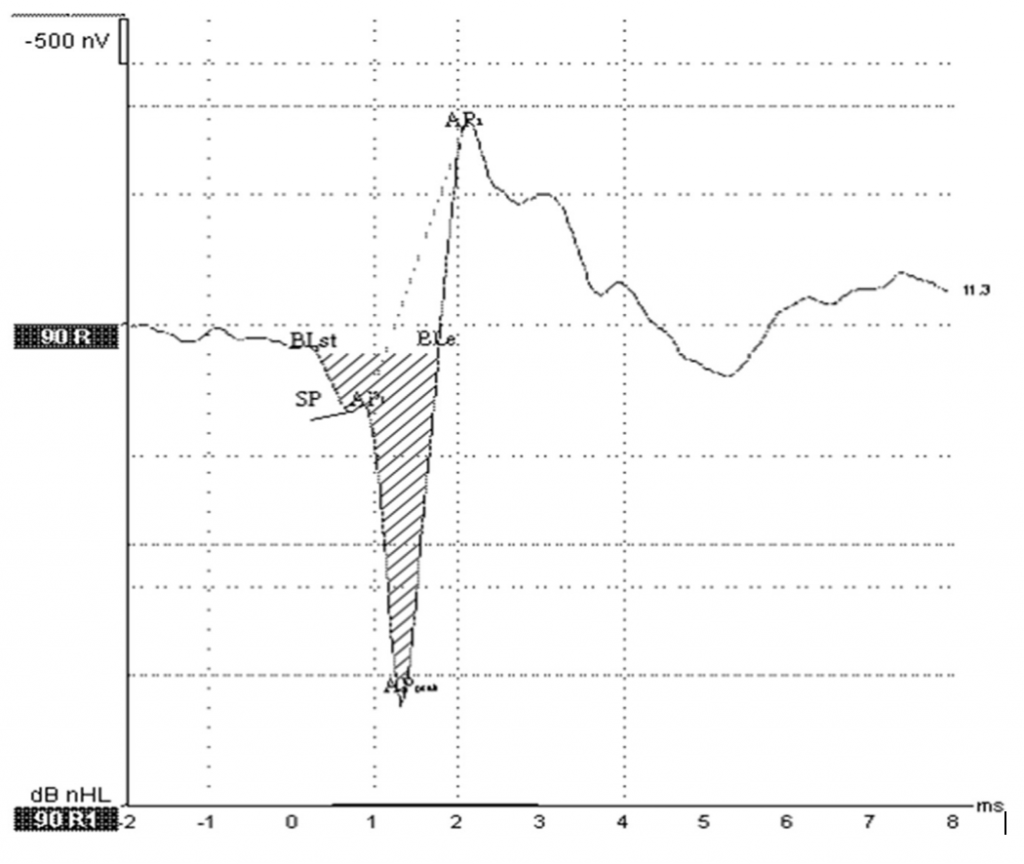
Figure 3. SP/AP area ratio. Grasel et al.6
As illustrated in Figure 4, the magnitudes of the SP and AP can be measured from peak to-trough (left panel), or with reference to a baseline value (right panel).
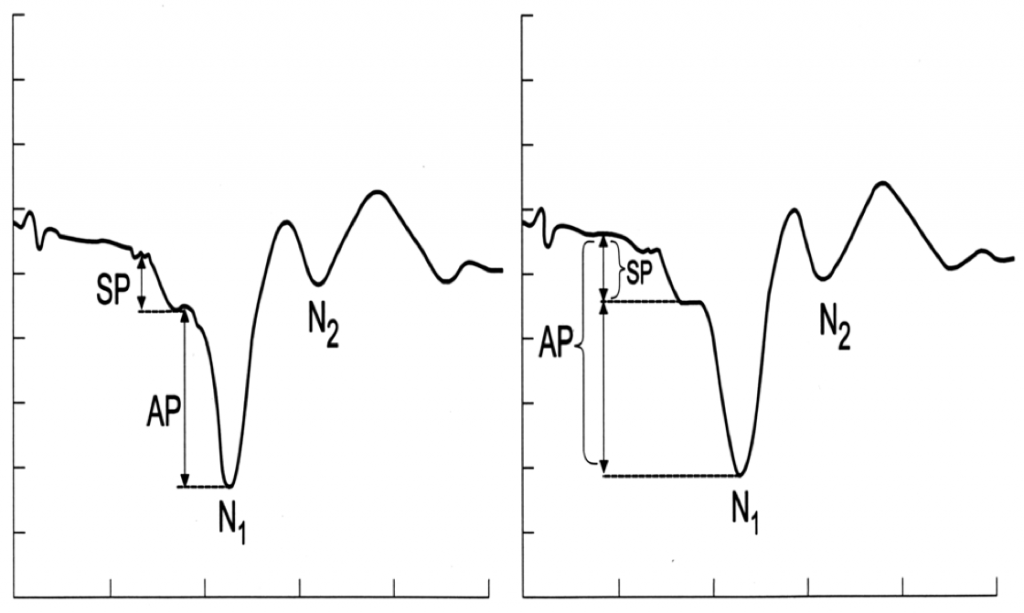
Figure 4. The magnitudes of the Summating Potential (SP) and Action Potential (AP) can be measured from peak to-trough (left panel), or with reference to a baseline value (right panel). From Ferraro.3
Measurements of the areas of the SP and AP to click stimuli to derive the SP/AP area ratio. In Figure 5, the shaded area on the left represent SP area, while shaded area on the right represents the AP area (Ferraro and Tibbils, 1999).2
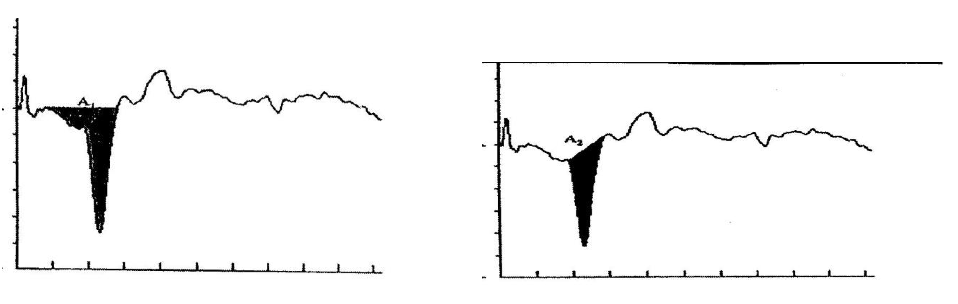
Figure 5. the shaded area on the left represent SP area, while shaded area on the right represents the AP area (Ferraro and Tibbils.2).
Despite the attractiveness of using the SP/AP area ratio in interpretation of ECochG, there is no consensus on how to calculate the SP and AP areas. It is advisable that each clinician interpreting ECochG should understand how his /her evoked potential system calculates SP/AP amplitude and area ratio. Each clinic is encouraged to collect its own normative data, and each Evoked Potential system manufacture should include an explanation of the software formula used for calculation of SP/AP amplitude and area ratio. Researchers should always highlight the used formula in quantifying their data. We believe that the increased utility of ECochG in different inner ear disorders mandates the presence of clinical guidelines for recording and interpretation of Electrocochleaography.
References
- Morrison AW, Moffat DA, and O’Connor AF. Clinical usefulness of electrocochleography in Ménière’s disease: An analysis of dehydrating agents. Otolaryngol Clin North Am 1980;11:703–21.
- Ferraro JA, Tibbils RP. SP/AP area ratio in the diagnosis of Ménière’s disease. Am J Audiol 1999;8:21–28.
- Ferraro JA. Electrocochleography. In: Roesser RJ, Valente M, Hosford-Dunn H, eds. Audiology: Diagnosis. New York: Thieme; 2000. 425–450.
- Ferraro J, Durrant J. Electrocochleography in the evaluation of patients with Ménière’s disease/endolymphatic hydrops. J Am Acad Audiol 2006;17:45–68
- Baba A, Takasaki K, Tanaka F, Tsukasaki N, Kumagami H, Takahashi H. Amplitude and area ratios of summating potential/action potential (SP/AP) in Ménière’s Disease, Acta Otolaryngol 2009;129(1):25–9.
- Grasel S. et al. Normative data for TM electrocochleography measures. J Otol 2017;12:68–73.
Suggested Reading
Al-Momani MO, Ferraro JA, Gajewski BJ, Ator G. Improved sensitivity of electrocochleography in the diagnosis of Ménière’s Disease. Int J Audiol 2009;48:811–19.
Dallos P, Schoeny ZG, Cheatham MA. Cochlear summating potentials: descriptive aspects. Acta Otolaryngologica 1972;301(Suppl.):1–46.
Devaiah AK, Dawson KL, Ferraro JA, Ator GA. Utility of area curve ratio electrocochleography in early Ménière’s disease, Arch Otolaryngol Head Neck Surg 2003 May;129(5):547–51.
Eggermont J. Ups and downs in 75 years of electrocochleography. Front Syst Neurosci 2017;11:2.
Margolis RH, Rieks D, Fournier MA, and Levine, SE. Tympanic electrocochleography for diagnosis of Meniere’s disease. Arch Otolaryngol–Head Neck Surg 1995;121:44–55
Pienkowski P, Adunka O, and Lichtenhan J. New advances in electocochleaography for clinical and basic investigations. Front Neurosci (2018: In press)

