Connecting Those with Hearing Loss to Surgical Centres When Hearing Aids Aren’t Enough
Introduction
The World Health Organization (WHO) predicts that by 2050, nearly 2.5 billion people will have some degree of hearing loss with approximately 700 million requiring hearing healthcare services (WHO, February 2, 2024). What remains to be determined is the proportion of individuals with hearing loss who will require implantable hearing devices. Clinicians not currently working with implantable devices may be asking themselves why this matters? The idealist would say, because all audiologists should strive to ensure that each client receives the best possible hearing care. This article outlines bone conduction and cochlear implant systems in general. Our goal is to clarify current criteria and device options for clinicians who do not work directly with implantable hearing devices. Please find here a summary of the key messages found in this article:
- Informally, the referral criteria can be summed up in the following ways. You should refer for a bone conduction device (BCD) consultation if your client has a permanent outer ear, middle ear, or ear canal disease or disorder that prevents them from successfully wearing standard air conduction hearing aids. This could include ear canal atresia, stenosis, chronic infection, or any issue causing a significant air-bone gap.
- New recommendations suggest that an individual should be referred for a cochlear implant consultation if they have a pure-tone average (PTA) of at least 60 dB and an unaided word recognition score of 60% or worse (known as the “60/60 rule”) in either ear. Individuals may have severe to profound hearing loss, steeply sloping loss, asymmetric hearing loss or single sided deafness (SSD). While not all individuals with these hearing configurations will pursue implantation, the referral to a CI team is appropriate.
- Lastly, a referral to an implantable hearing device team does not mean an individual must proceed with surgery. Referring to these facilities helps inform patients of the healthcare options available to them and helps determine the best treatment option. Clinician’s working with implantable hearing devices do not want to poach your patients – in fact, they will likely become your partner in hearing healthcare.
Bone Conduction Implants
When conductive or mixed hearing loss is confirmed, patients will first be referred to an ENT specialist to look at potential medical or surgical treatments that may reverse the condition. If the hearing loss persists, one of the roles of the audiologist is to determine if a hearing aid is appropriate or if a bone conduction device (BCD) could prove a better solution.
Indications for Bone Conduction Devices
Traditionally, BCDs were prescribed to patients who presented with an anatomy that could not accommodate conventional hearing aids. Cases included atresia, microtia and other congenital outer or middle ear issues. BCDs are also a great option for patients with chronic middle ear issues (e.g., discharge). Unlike traditional hearing aids, BCDs amplify without occluding the external auditory canal thus minimizing moisture accumulation and decreasing the risk of infections (Backous et al., 2022).
Bone conduction devices are also viable for mixed losses with a significant conductive component. Patients with an air-bone gap greater than 30 dB often perform better with a BCD than a traditional hearing aid (de Wolf MJ et al., 2011).
In a small number of cases, a BCD can also serve as a solution for individuals with single-sided deafness (SSD) who are not suitable candidates for a contralateral routing of signal (CROS) system or cochlear implantation . The device functions similarly to a CROS system, with the key difference being that the sound signal is transmitted to the cochlea of the better-hearing ear via bone conduction. A BCD trial may be offered if a patient is not satisfied with a CROS hearing aid.
Candidacy Criteria
Bone Conduction Devices are indicated for patients with conductive or mixed hearing loss, an air-bone gap of at least 30 dB, and good speech discrimination scores (Sanchez-Perez J, 2023). Pure-tone bone conduction thresholds should not exceed 55 dB HL. However, the healthier the cochlea (i.e., the better the bone conduction thresholds), the better the outcomes will be. Since BCDs bypass the outer and middle ear and stimulate the cochlea directly, outcomes are closely linked to cochlear health. As a patient’s bone conduction thresholds worsen (e.g., presbycusis), the benefit from a BCD decreases. In cases of progressive hearing loss, a BCD may not be able to provide adequate gain and clarity may decrease over time. Other alternatives (such as CI) may be considered in these situations.
A trial with a BCD fit on a softband or headband is typically conducted for potential candidates. While soft band or headband trials can be done in the clinic patients should trial a device at home to evaluate its effectiveness, benefits, and limitations. A trial at home helps patients develop more realistic expectations and improves BCD acceptance.

Examples of Good BCD Candidates
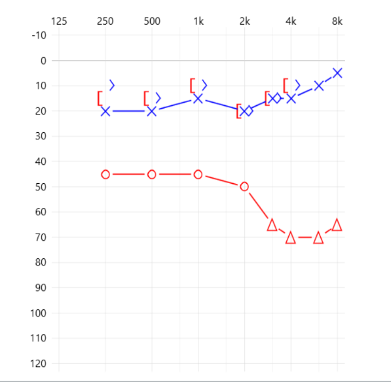
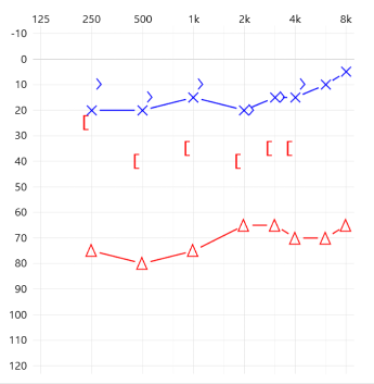
Assuming these patients have good word discrimination scores, they should be considered candidates for BCD on their right ear as they present with more than a 30 dB air-bone gap and an average BC threshold that fits most manufacturers’ specifications.
Types of Bone Conduction Devices
To choose the appropriate BCD for a patient, an audiologist will consider the patient’s audiogram, conduct an evaluation of needs, and take their medical health into account. There are two main categories of BCDs: passive and active devices. These categories refer to how the bone conduction vibration stimulation is delivered to the cochlea. Passive or over-skin drive devices transmit vibrations through the skin to the bone. In contrast, active or direct bone drive devices apply vibrations directly to the bone. These are broken down into further sub-categories depending on the processor’s attachment.
Passive, Non-Surgical Bone Conduction Devices
Bone Conduction Devices do not always require surgery. Depending on the manufacturer, processors can be secured to the head using several methods, including soft, stretchy headbands, metal or plastic headbands, or adhesive stickers. Clinicians should remember that the functional output of non-surgical BCDs varies with skin and hair thickness.
Passive non-surgical BCDs are often used in the following cases:
- <5 years of age;
- for patients with fluctuating conductive hearing loss;
- patients with insufficient bone density or poor bone quality;
- and for those who cannot or do not wish to undergo surgery.
Active Percutaneous
Active percutaneous BCDs utilize a surgically implanted, osseointegrated screw with a connected abutment that protrudes through the skin. The abutment is used as the coupling mechanism for the sound processor. Proper care of the abutment is essential in maintaining the integrity of the surrounding tissues. Complications associated with poor care and maintenance have led to percutaneous devices becoming a less popular treatment option. However, percutaneous devices may be preferred when there are concerns regarding the surgical process. Specifically, the abutment placement can be completed in less than one hour and under local anesthetic. Consequently, active percutaneous devices may suit patients who cannot undergo general anesthesia or prefer less invasive surgery (Wojciech, 2022). The fitting range for percutaneous devices is also wider than that of transcutaneous devices meaning individuals with poorer bone lines can still receive treatment.
Passive Transcutaneous
Passive transcutaneous BCDs also utilize a surgically implanted osseointegrated screw but connect to an internal magnet rather than an abutment. The sound processor attaches to an external magnet which is used to transmit sound to the internal magnet and implant. As with non-surgical options, the functional output of passive transcutaneous devices is impacted by skin and hair thickness.
Active Transcutaneous
Active transcutaneous devices consist of a surgically implanted transducer directly vibrating the bone and a magnetically connected external sound processor. Patients typically experience better sound quality with fewer skin complications, making this device the preferred choice for many patients.
When you encounter an individual with a significant air-bone gap struggling to wear or who cannot wear conventional hearing aids, please consider referring them to your local bone conduction device team. Referrals do not need to specify the exact style of BCD to be used as that can be left up to the specialist team (see Appendix I for list of centers across Canada working with bone-conduction implants).
Cochlear Implants
Evidence from the literature suggests penetration rates for cochlear implantation is less than 13% of eligible adults in North America (Sorkin, 2013; Nassiri, 2022) and less than 60% of eligible children in North America (Sorkin & Buchman, 2016). While there are many reasons for this, one contributing factor is a generally low rate of referrals from audiologists to cochlear implant programs. Contemporary indications for referral for a cochlear implant evaluation are provided below.
A useful shorthand for when to refer for cochlear implant assessment is 60/60 referral guideline (Zwolan, 2020). The 60/60 referral guideline suggests that an individual should be referred for cochlear implant evaluation when the pure-tone average is 60 dB HL or worse and unaided monosyllabic word scores are less than 60% correct. Following this guideline promotes timely access to cochlear implant surgery with the potential for improved hearing outcomes. This is because a significant contributing factor to performance with a cochlear implant is neural health. When cochlear implantation occurs closer in time to when an individual no longer benefits from a hearing aid in the ear being implanted, their neural health is likely to be better.
The cochlear implant candidacy evaluation process is generally consistent across all patient types. After your referral to a cochlear implant centre, the audiologist will do additional testing to confirm auditory function with an appropriately fit hearing aid. They will also review the individual’s hearing history, focusing on duration of deafness and consistency of hearing aid use. Additionally, there will be a medical review to assess the anatomy of the inner and middle ears as well as the viability of the cochlear nerve.
Cochlear implant candidacy assessments can be overwhelming for the person undergoing evaluation. Their hopes are often very high, and they may be desperate for any option that helps them to hear. As a clinician working with individuals with bilateral severe-to-profound hearing loss, it is important that you do not think of cochlear implantation as the “last stop” or a “last ditch effort” in the hearing journey. All professionals who have been a part of someone’s hearing journey help to shape the perspectives of the individual seeking this line of treatment. As hearing health care advocates, audiologists should promote cochlear implantation through a positive lens for individuals who no longer benefit from hearing aids. You may be wondering how to counsel clients in a supportive way. Here we share one supportive conversation heard during a Cochlear Implant Evaluation as recounted from a client:
“My hearing aids were not working anymore, and [my Audiologist] said to me: Your hearing loss has progressed in a way that only allows hearing aid(s) to help so much. There are other options that can support your communication and hearing needs. I am not able to help you in the next part of your journey. I would like to connect you with an Audiologist who can guide you through the next steps of your journey. They can help you to better understand how a cochlear implant might help you moving forward.”
Anecdotally, clinics that review their client base for possible cochlear implant referrals are apt to find an increase in revenue by doing so. Clients referred for cochlear implant candidacy often need or choose to proceed with, a new hearing aid purchase instead of, or in addition to, cochlear implantation. With the array of hearing aids and assistive listening devices compatible with implantable devices, referring clinics are also likely to see additional sales even for clients who proceed with implantation.
Traditional Candidacy (Unilateral or Bilateral Cochlear Implantation)
Traditional cochlear implant candidacy is defined by bilateral severe-to-profound sensorineural hearing loss with extremely poor word recognition scores. While traditional candidacy criteria are often well known in the Audiological community, contemporary indications have expanded the number of cases that might benefit from a cochlear implant. Changes to candidacy criteria are driven by successes in individuals who received their implant while being outside the “traditional” criteria. These contemporary indications are often less understood by clinicians working outside of cochlear implant centres and, depending on your location in Canada may or may not be supported under the provincial or territorial health funding agency.

Asymmetric Hearing Loss/Bimodal Candidacy
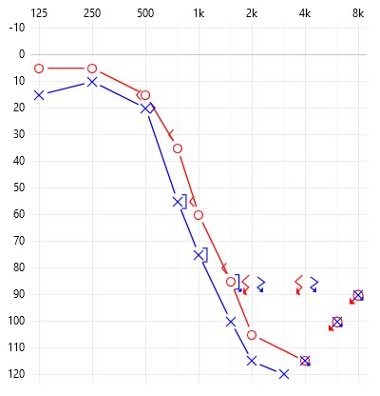
Contemporary indications for cochlear implantation stress the importance of assessing each ear individually to provide optimal access to sound in both ears. Thus, if an individual has one ear where the PTA is 60 dB HL or worse and their unaided word recognition scores are below 60%, a referral to a CI centre is indicated.
Example of word recognition scores
RIGHT WRS: 60% at 85 dB HL
LEFT WRS: 0% at 105 dB HL
When access to Cochlear Implantation is timely, and met by the recipient with appropriate motivation and expectations, performance with bimodal hearing can be excellent. Individuals using a hearing aid and cochlear implant together benefit from assistive devices in a new way. These individuals often report improvements in functional hearing in challenging listening environments (e.g., noisy environments, echoic spaces) and when listening over the phone. These hearing benefits require synergies between the team of hearing healthcare providers working with the individual. This creates opportunities for the community-based audiologist to continue working with long-term clients while collaborating with the cochlear implant audiologist.
Electric Acoustic System (EAS) Candidacy
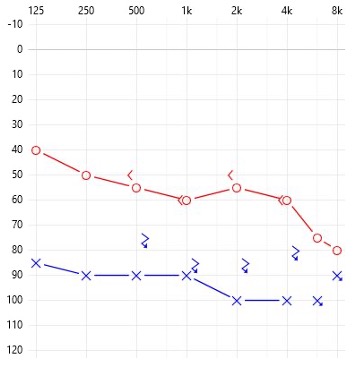
Electric Acoustic Systems (EAS) are for severe “ski-slope” hearing loss patients. In cases where frequency lowering technology is not beneficial (i.e., cannot provide adequate speech clarity) referral to a cochlear implant program for EAS evaluation is appropriate. A combination of new surgical techniques and innovative cochlear implant array design have allowed for the preservation of hearing such that the sound processor can provide both acoustic and electric outputs in some cases. For individuals with severe ski-sloping hearing loss, acoustic output can be used in the good/better hearing low-frequencies while the electric signal can provide access to information above approximately 500 Hz to 1000 Hz, depending on the hearing loss. Functionally, these individuals use a hearing aid and a cochlear implant in the same ear providedidual hearing is preserved post-operatively. Candidacy criteria for EAS are similar to those for asymmetric hearing loss, with greater emphasis placed on very poor word discrimination scores as the trigger for referral.
EXAMPLE OF EAS SPEECH PERFORMANCE
RIGHT WRS: 36% at 75 dB HL
RIGHT AIDED PERFORMANCE
AzBio (65 dB SPL): 50%
AzBio (65 dB SPL) + 5 dB SNR: 10%
CNC Words: 0%; Ph: 30%
LEFT WRS: 24% at 75 dB HL
LEFT AIDED PERFORMANCE
AzBio (65 dB SPL): 40%
AzBio (65 dB SPL) + 5 dB SNR: 2%
CNC Words: 0%; Ph: 4%
Single-Sided Deafness (SSD) Candidacy
The newest indication for cochlear implant candidacy is single-sided deafness (SSD). Health Canada approved This indication as a treatment option for those with severe-to-profound sensorineural hearing loss in one ear and normal hearing in the contralateral ear in 2020. Indications around the duration of deafness in the ear to be implanted are still being explored, but clinical experience suggests that the shorter the duration of hearing loss is, the better the outcomes will be. Individuals with this configuration of hearing loss may be offered a CROS system or BAHA to see if either option meets their hearing needs. The potential benefits of cochlear implantation for SSD are the ability to restore hearing in the deafened ear itself, to restore a sense of “balance” between the patient’s ears, to improve understanding in noisy environments, to reduce listening effort, and to potentially reduce tinnitus severity.
When presented with a patient struggling with their current hearing technology who you believe may benefit from cochlear implantation, please refer to your local cochlear implant centre. Once again, referral to a cochlear implant team does not guarantee an individual will proceed with surgery. It is simply to allow patients to learn about the options that could potentially improve their hearing function and quality of life (see Appendix II).
Conclusion
Our duty as audiologists is to ensure that every person we see is allowed the best hearing possible. In some cases, our best efforts with conventional technology fall short of the patient’s needs. In cases like those discussed above, we invite you to refer to an implantable hearing technology team. While implantable devices do not “cure” or fully resolve all issues, they may substantially improve an individual’s quality of life. The clinicians you refer to look forward to being your partners in hearing healthcare.
Appendix I - Canadian Centres Providing Bone-Conduction Implant Surgery
Eligibility for funding of these devices may vary by province. Funding supports may impact candidacy criteria for a given region.
| Province | Centre | Contact Information | Website |
| Newfoundland | Eastern Health Audiology Department, General Hospital 300 Prince Philip Drive St. John’s, NL A1B 3V6 | Ph: 709-777-7943 Fax: 709-777-7942 | N/A |
| Nova Scotia | Nova Scotia Hearing and Speech Centres (QEII) 5820 University Ave., #rd Floor, Room 3084 Halifax, NS B3J 3R4 | Ph: 902-473-4349 | Hearing testing & diagnosis | Hearing & Speech Nova Scotia |
| Nova Scotia Speech and Hearing (IWK) 5919 South Street PO Box 9700 Halifax, NS B3K 6R8 | Ph: 902-470-8049 | Hearing testing & diagnosis | Hearing & Speech Nova Scotia | |
| Québec | CHU de Québec, l’Hôtel Dieu de Québec Centre Québecois d’expertise en implant cochléaire Services d’audiologie 11 Côté du Palais Québec, QC G1R 2J6 | (Adulte)Ph : 418-691-5407 Fax : 418-691-5377 (Pédiatrie) 418-654-2116 | https://www.chudequebec.ca/professionnels-de-la-sante/formulaires-et-references/apercu/formulaire-de-reference-en-audiologie.aspx |
| CHUM 1000 rue St.-Denis, Pavillion C, 9e étage, Montréal, Québec | Ph : 514-890-836 | www.chumontreal.qc.ca | |
| Ste.-Justine 3175 Chemin de la Côte Ste-Catherine Montréal, QC H3T 1C5 | Ph : 514-354-4612 | Audiologie pour les enfants | CHU Sainte-Justine | |
| CUSM – McGill Health Science Centre – Royal Victoria Hospital 1001 boul. Décarie Montréal, QC H4A 3&1 | Ph: 514-934-8028 | ||
| CUSM- McGill Health Science Centre – Montreal Children’s Hospital 1001 Boul Décarie, A.R.C.4227 Montréal, QC H4A 3J1 | Ph : 514-412-4454 | Audiology - Montreal Children’s Hospital | |
| Ontario | The Ottawa Hospital, Civic Campus (Adults) Audiology Department 737 Parkdale Ave., Room 248 Ottawa, ON K1Y 1J8 | Ph: 613-798-5555 X 18003 Fax; 613-761-4312 | |
| Children’s Hospital of Eastern Ontario (Children) Audiology Services Clinic B, 401 Smyth Road Ottawa, ON K1H 8L1 | Ph: 613-737-7600 Fax: 613-738-4222 | ||
| Hotel Dieu (Kingston Health Sciences Centre) 75 Stuart St. Kingston, ON K7L 2V7 | Ph: 613-544-3400 x 3623 | ||
| Markham Hearing Centre Box Grove Medical Arts Centre 110 Copper Creek Dr., Suite 105 Markham, Ontario L6B 0P9 | Phone: 905-471-4479 Fax: 905-472-5436 Email: info@markhamhearing.ca | https://markhamhearing.ca/services/baha-program | |
| Sunnybrook Health Sciences Centre (Adults) M1-102, 2075 Bayview Ave. Toronto, ON M4N 3M5 | Ph: 416-480-6751 Fax: 416-480-5761 | ||
| Hospital for Sick Children (Children) 555 University Ave. Toronto, ON M5G 1X8 | Ph: 416-813-7259 Fax: 416-813-5036 | ||
| Queensway Professional Centre 101 Queensway West, Suite 102 Mississauga, ON L5B 2P7 | Ph: 289-232-0935 Fax: 905-277-4439 | ||
| London Health Sciences Centre University Hospital, Main Floor 339 Windermere Road London, ON N6A 5A5 | Ph: 519-663-3641 Fax: 519-663-3916 | ||
| Manitoba | Surgical Hearing Implant Program Health Sciences Centre GB421 – 820 Sherbrook St. Winnipeg, MB R3T 4J6 | Ph: 204-787-5039 Fax: 204-787-5109 | https://entmanitoba.ca/services/surgical-hearing-program/contact-information |
| Central Speech and Hearing Clinic Unit 2- 1325 Markham Road Winnipeg, MB R3T 4J6 | Ph: 204-787-5039 Fax: 204-787-5109 | https://centralspeech.ca/our-programs-and-services/adult-cochlear-implant-program | |
| Saskatchewan | Royal University Hospital Room 25, Ellis Hall 103 Hospital Dr. Saskatoon, SK S7N 0J9 | Ph: 306-655-0989 Fax: 306-655-1316 | N/A |
| Alberta | Glenrose Rehabilitation Hospital 10230 – 111th Ave. Edmonton, AB T5G 0B7 | Ph: 780-735-7945 Fax: 780-735-6031 | https://www.albertahealthservices.ca/find/health/Service.aspx?id=4185&serviceAtFacilityID=1005480 |
| Edmonton Bone Conduction: iRSM (adults and pediatrics) 1W-02, 16940-87 Ave. Edmonton, AB T5R4H5 | Ph: 780-735-2660 Fax: 780-735-2658 | https://www.irsmyeg.ca/hearing-solutions/ | |
| Alberta Children’s Hospital 2888 Shaganappi Trail NW Calgary, AB T3B 6A8 | Ph: 403-955-7061 Fax: 403-955-2674 | https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1000912&serviceAtFacilityID=1-23259 | |
| Calgary Adult Bone Conduction: South Health Campus | https://www.albertahealthservices.ca/find/health/Service.aspx?id=1084251&serviceAtFacilityID=1133551 | ||
| Community Audiology Richmond Road Diagnostic and Treatment Centre (RRDTC) – Adult Site 1820 Richmond Road SW Calgary, AB T2T 5C7 | Ph: 403-955-8500 Fax: 403-955-8501 | https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1010213&serviceAtFacilityID=1023523 | |
| British Columbia | BC Adult Cochlear implant Program St. Paul’s Hospital Rm. 2618, Providence Building 1081 Burrard St. Vancouver, BC V6Z 1Y6 | Ph : 604-806-9616 Fax : 604-806-8435 | https://www.providencehealthcare.org/en/clinics/bc-adult-cochlear-implant-program |
| BC Children’s Hospital 4480 Oak St. Cochlear Implant Services, RM 1D-20 Vancouver, BC V6H 3V4 | Ph : 604-875-2345 Fax : 604-875-2977 | https://www.bcchildrens.ca/our-services/clinics/bone-conduction-implants | |
| Kelowna General Hospital 2268 Pandosy St., Kelowna BC V1Y 1T2 | Ph: 250-862-4000 Fax: 250-862-4020 | https://www.interiorhealth.ca/locations/kelowna-general-hospital | |
| Jim Pattison Outpatient Care and Surgery Centre, 9750- 140th St., Surrey, British Columbia, V3T 0G9 (604)582-4550. |
Appendix II - Canadian Centres Implanting and/or Providing Care for Cochlear Implant Recipients
Eligibility for funding of these devices may vary by province. Eligibility for a given region may be impact candidacy criteria.
| Province | Centre | Contact Information | Website |
| Newfoundland | Eastern Health Audiology Department, General Hospital 3000 Prince Philip Drive St. John’s, NL A1B 3V6 | Ph: 709-777-7943 Fax: 709-777-7942 | N/A |
| New Brunswick | Hôpital Régional Chaleur/Regional Hospital Réseau de Santé Vitalité Health Network 1750 Sunset Drive Bathurst, NB #2A 5L7 | Ph : 506-544-3869 Fax : 506-544-3431 | https://www.vitalitenb.ca/fr/points-de-service/hopitaux/centre-hospitalier-universitaire-dr-georges-l-dumont/audiologie |
| Nova Scotia | Nova Scotia Hearing and Speech Centres 5667 Spring Garden Road, Park Lane Terraces Suite 201, Box 112, Halifax, NS B3J 3R4 | Ph: 902-492-8201 Fax: 902-423-0981 | https://www.hearingandspeech.ca/our-services/hearing |
| Québec | CHU de Québec, l’Hôtel Dieu de Québec Centre Québecois d’expertise en implant cochléaire Services d’audiologie 11 Côté du Palais Québec, QC G1R 2J6 | Ph : 418-691-5420 Fax : 418-691-5377 | |
| CIUSS de la Capitale Nationale IRDPQ Institut de réadaptation en déficience physique de Québec 2975 Chemin St. Louis Québec, QC G1W 1P9 | Ph : 418-529-9141 Fax : 418-626-3914 | ||
| Université Laval Programme d’audiologist/ Faculté de médecine 1050, ave. de la Médicine, Bureau 4455 Québec, QC G1V 0A6 | Ph : 418-656-2131 x 412684 | https://www.chudequebec.ca/patient/maladies-soins-et-services/specialites-et-specialistes/specialites/implant-cochleaire.aspx | |
| CIUSSS du Centre-Sud-de-l’Île-de-Montréal, Institut Raymond-Dewar Clinique de programmation de l’implant cochléaire 2222 Laurier Est Montréal, QC H2H 1C4 | Ph : 514-284-2581 Fax : 514-284-5086 | ||
| Montreal Oral School for the Deaf 4670 Ste. Catherine St. West Westmount, QC H3Z 1S5 | Ph: 514-488-4946 Fax: 514-488-5398 | https://montrealoralschool.com/en/programs-and-services/ | |
| CIUSS du Centre-Ouest-de-l’Île-de-Montréal Lethbridge-Layton-Mackay Rehabilitation Centre 7000 Sherbrooke St., Ouest Montréal, QC H4B 1R3 | Ph : 514-488-5552 Fax : 514-489-3477 | ||
| McGill University Health Centre Cochlear implant Program Department of Speech-Language Pathology and Audiology D04 7510 Glen Campus 10001 boul. Décarie Montréal, QC H4A 3&1 | https://muhc.ca/cochlear-implant | ||
| Ontario | The Ottawa Hospital, Civic Campus (Adults) Audiology Department 737 Parkdale Ave., Room 248 Ottawa, ON K1Y 1J8 | Ph: 613-798-5555 X 18003 Fax; 613-761-4312 | https://www.ottawahospital.on.ca/en/clinical-services/deptpgrmcs/departments/audiology/ |
| Children’s Hospital of Eastern Ontario (Children) Audiology Services Clinic B, 401 Smyth Road Ottawa, ON K1H 8L1 | Ph: 613-737-7600 Fax: 613-738-4222 | https://www.cheo.on.ca/en/clinics-services-programs/audiology-clinic.aspx#:~:text=Our%20program%20provides%20a%20full,floor%20of%20CHEO’s%20main%20campus. | |
| Sunnybrook Health Sciences Centre (Adults) M1-102, 2075 Bayview Ave. Toronto, ON M4N 3M5 | Ph: 416-480-6751 Fax: 416-480-5761 | https://sunnybrook.ca/content/?page=cochlear-implant-program-information | |
| Hospital for Sick Children (Children) 555 University Ave. Toronto, ON M5G 1X8 | Ph: 416-813-7259 Fax: 416-813-5036 | https://www.sickkids.ca/en/care-services/clinical-departments/otolaryngology/ | |
| London Health Sciences Centre University Hospital, Main Floor 339 Windermere Road London, ON N6A 5A5 | Ph: 519-663-3641 Fax: 519-663-3916 | https://www.lhsc.on.ca/cochlear-implant-program/about-us | |
| Manitoba | Surgical Hearing Implant Program Health Sciences Centre GB421 – 820 Sherbrook St. Winnipeg, MB R3T 4J6 | Ph: 204-787-5039 Fax: 204-787-5109 | https://entmanitoba.ca/services/surgical-hearing-program |
| Central Speech and Hearing Clinic Unit 2- 1325 Markham Road Winnipeg, MB R3T 4J6 | Ph: 204-787-5039 Fax: 204-787-5109 | https://www.centralspeech.ca/ | |
| Saskatchewan | Royal University Hospital Room 25, Ellis Hall 103 Hospital Dr. Saskatoon, SK S7N 0J9 | Ph: 306-655-0989 Fax: 306-655-1316 | https://www.saskhealthauthority.ca/facilities-locations/royal-university-hospital |
| Alberta | Glenrose Rehabilitation Hospital 10230 – 111th Ave. Edmonton, AB T5G 0B7 | Ph: 780-735-7945 Fax: 780-735-6031 | https://www.albertahealthservices.ca/findhealth/Service.aspx?id=4185&serviceAtFacilityID=1005480 |
| Alberta Children’s Hospital 2888 Shaganappi Trail NW Calgary, AB T3B 6A8 | Ph: 403-955-7061 Fax: 403-955-2674 | https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1000912&serviceAtFacilityID=1023259 | |
| Community Audiology Richmond Road Diagnostic and Treatment Centre (RRDTC) – Adult Site 1820 Richmond Road SW Calgary, AB T2T 5C7 | Ph: 403-955-8500 Fax: 403-955-8501 | https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1010213&serviceAtFacilityID=1023523 | |
| British Columbia | BC Adult Cochlear implant Program St. Paul’s Hospital Rm. 2618, Providence Building 1081 Burrard St. Vancouver, BC V6Z 1Y6 | Ph : 604-806-9616 Fax : 604-806-8435 | https://www.providencehealthcare.org/en/clinics/bc-adult-cochlear-implant-program |
| BC Children’s Hospital 4480 Oak St. Cochlear Implant Services, RM 1D-20 Vancouver, BC V6H 3V4 | Ph : 604-875-2345 Fax : 604-875-2977 | http://www.bcchildrens.ca/health-professionals/refer-a-patient/cochlear-implant-referral |
References
- Backous D, Choi BY, Jaramillo R, Kong K, Lenarz T, Ray J, Thakar A, Hol MKS. Hearing Rehabilitation of Patients with Chronic Otitis Media: A Discussion of Current State of Knowledge and Research Priorities. J Int Adv Otol. 2022 Jul;18(4):365-370. doi: 10.5152/iao.2022.21428. PMID: 35894534; PMCID: PMC9404322.
- De Wolf MJ, Hendrix S, Cremers CW, Snik AF. Better performance with bone‐anchored hearing aid than acoustic devices in patients with severe air‐bone gap. Laryngoscope. 2011;121:613-616.
- Dhanasingh, A. &. (2021). EAS-Combined electric and aocustic stimulation. . Acta oto-Laryngologica, 141(S1), 22-62. doi:10.1080/00016489.2021.1888477
- Hol, M.K.S., Kunst, S.J.W., Snik, A.F.M. et al. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Otorhinolaryngol 267, 889–896 (2010). https://doi.org/10.1007/s00405-009-1147-9
- Nassiri, A. S. (2022). Current Estimates of Cochlear Implant Utilization in the United States. Otol Neurotol., 43(5), e558-e562. doi:10.1097/MAO.0000000000003513
- Organization, W. H. (February 2, 2024). ReporT: Deafness and Hearing Loss.
- Sanchez-Perez J, Riera March A. Osseointegrated Bone-Conducting Hearing Protheses. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564385/
- Sorkin, D.L. (2013). Cochlear implantation in the world’s largest medical device market: utilization and awareness of cochlear implants in the United States. Cochlear Implants Int. Mar 14: Suppl 1.: S4-12. doi: 10.11179/1467010013z00000000076.
- Sorkin, D.L. & Buchman, C.A. (2016). Cochlear Implant Access in Six Developed Countries. Otol Neurotol. 37(2): e161-4. doi: 10.1097/MAO.0000000000000946.
- Wendrich, Anne W.; Kroese, Tiuri E.; Peters, Jeroen P. M.; Cattani, Guido; Grolman, Wilko. Systematic Review on the Trial Period for Bone Conduction Devices in Single-Sided Deafness: Rates and Reasons for Rejection. Otology & Neurotology 38(5):p 632-641, June 2017. | DOI: 10.1097/MAO.0000000000001405 https://pubmed.ncbi.nlm.nih.gov/28414693/
- Wojciech Gawęcki, Renata Gibasiewicz, Joanna Marszał, Magdalena Błaszczyk, Maria Gawłowska, Małgorzata Wierzbicka, The evaluation of a surgery and the short-term benefits of a new active bone conduction hearing implant - the Osia®, Brazilian Journal of Otorhinolaryngology, Volume 88, Issue 3, 2022,Pages 289-295, ISSN 1808-8694, https://doi.org/10.1016/j.bjorl.2020.05.021.
- Zwolan, T. S.-L. (2020). Development of a 60/60 Guideline for Referring Adults for a Traditional Cochlear Implant Candidacy Evaluation. Otol Neurotol., 41(7), 895-900. doi:10.1097/MAO.00000000002664