To the Brain and Back: Can Stress Cause Tinnitus and Hyperacusis?
Tinnitus and hyperacusis are highly stressful conditions. People with tinnitus report higher stress levels than those without tinnitus,1 and stress can make tinnitus louder and more distressing.2 Stress reduction is often recommended for effective management of tinnitus, as noted almost ten years ago in an article written by Jennifer Gans for Canadian Audiologist.3
There is a wide belief that stress itself can cause tinnitus or hyperacusis, even when hearing ability is normal. Since the 1800s, clinicians have observed that patients report psychological stress before and during the development of these conditions.4 Public health research also shows that stress increases the risk of tinnitus as much as noise exposure.5 To date, no study has clearly demonstrated that stress per se initiates tinnitus and hyperacusis in humans, but researchers have long suspected that causal connections exist. In this article, I will discuss several ways that stress could lead to the development of auditory disorders. Tinnitus will receive the most focus, however, research on hyperacusis will also be considered.
The body’s response to stress
The physiological stress response is complex and involves interactions between endocrine, nervous, and immune systems.6 These systems help the body react and adapt to stressors, which can include emotional, physical, or environmental challenges. In the endocrine system, stress activates the hypothalamic-pituitary-adrenal (HPA) axis, a chain reaction between brain regions and glands in the body that leads to the secretion of stress-related hormones, such as cortisol, into the bloodstream (Figure 1). Cortisol performs many roles, but a major function is to regulate the body’s energy use. Under normal circumstances, the HPA response is short-lived and operates through a negative feedback loop—stress activates the HPA axis, and the subsequent cortisol release terminates the HPA response. In humans, cortisol levels can be measured directly from saliva samples.
Stress also activates the sympathetic nervous system, the well-known fight or flight response. Its hallmarks include increased blood pressure, respiration, vasoconstriction, sweating, pupil dilation, and slowed digestion. Sympathetic activation is accompanied by the release of epinephrine (also known as adrenaline) and norepinephrine from the adrenal gland. Like the HPA axis, the sympathetic response is a closed-loop negative-feedback system that is terminated by epinephrine. Physiological measures such as skin conductance, heart rate monitoring, and body temperature can track sympathetic activation. Alpha-amylase, an enzyme released from salivary glands, is another way to track sympathetic stress responses.
The immune system is also involved in the body’s stress response. Cortisol and epinephrine from the HPA axis and sympathetic nervous system influence how immune cells and cytokines (proteins involved in inflammation responses) are released and distributed through the body. HPA and sympathetic activation often suppress the immune system, but under specific conditions, stress can have a stimulating effect. Cytokine levels can be measured from blood samples.
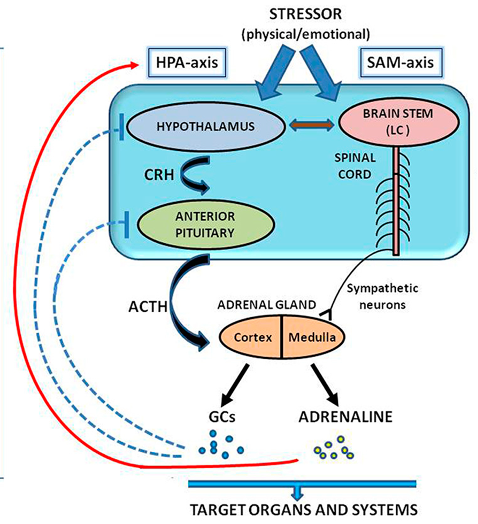
Figure 1 Stress pathways. On the left, stress activates the hypothalamic-pituitary-adrenal (HPA) axis. The response initiates in the hypothalamus, where corticotropin-releasing hormone (CRH) causes adrenocorticotropic hormone (ACTH) to be released by the pituitary gland. ACTH causes the release of glucocorticoids (GCs) from the adrenal gland, which includes cortisol. Cortisol terminates the HPA response (dashed blue lines). On the right, sympathetic activation, here referred to as the sympathetic-adrenal-medullar (SAM) axis, originates in the brainstem and leads to a release of epinephrine (adrenaline) from the medulla of the adrenal gland. The release of epinephrine, with cortisol and other glucocorticoids, affects several organs and bodily systems. Importantly, these systems (alongside the body's immune system) interact with one another. This image is adapted from Baritaki, S., de Bree, E., Chatzaki, E., & Pothoulakis, C. (2019). Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. Journal of Clinical Medicine, 8(10), 1669,7 and is shared by terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
The effect of stress on each system has several potential consequences for auditory function. Importantly, these effects can arise from environmental or psychological stressors, not just auditory stressors like noise exposure. As mentioned, short-term “acute” stress can help the body and brain to adapt to challenges and build resilience. In fact, short-term stress can protect the auditory system against noise damage. 8,9 However, long-term stress can be detrimental to bodily function. Chronic stress disrupts HPA function, and cortisol levels increase without disengaging the HPA response. Sympathetic nervous system activity (fight or flight) similarly increases, and the immune system can become dysfunctional, leading to increased inflammation throughout the body. Research shows that chronic stress is associated with a variety of undesirable health outcomes, such as hypertension, heart disease, sleep problems, weight gain, disrupted digestion, muscle tension, and anxiety and depression.10 In the auditory system, repeated exposure to stressors negatively affects neural processing and perception of sound.11
Stress, tinnitus, and hyperacusis
Hearing damage is widely presumed to precipitate tinnitus and hyperacusis. When cochlear sensory cells are compromised, downstream neurons in the central auditory system become hyperactive, resulting in “ringing of the ears” or abnormal loudness sensitivity. Several studies show that the presence of tinnitus is associated with dysregulation of stress systems. For example, tinnitus sufferers show abnormal cortisol levels and attenuated short-term cortisol responses to psychological or noise stressors.12,13 One study in men with tinnitus also showed increased alpha-amylase in saliva, evidence of elevated sympathetic activity.14
These studies are correlational, meaning they cannot tell us about the presence or direction of causal relationships. However, there are multiple ways that chronic stress could result in these disorders. First, chronic stress response itself may damage the cochlea, providing a route to hyperacusis and tinnitus similarly to noise damage and age-related decline. Second, stress may modulate central auditory activity downstream of the cochlea, which could initiate or contribute to neural hyperactivity. In these cases, stress may create conditions for tinnitus and hyperacusis, even when hearing ability is normal. A third explanation is that perceived stress exacerbates noise injury or other insults to the auditory system.
Direct effects of stress on cochlear function
Can stress itself cause damage to the cochlea? Szcepcak and Mazurek detail several ways that dysfunction in stress pathways could produce this outcome.15 For instance, chronic stress can damage mitochondria and disrupt gene transcription in cochlear hair cells, leading to cell death. Stress may also cause cochlear loss through glutamate excitotoxicity. 16 Glutamate excitotoxicity is usually associated with noise-induced hearing loss. Glutamate is the primary excitatory neurotransmitter in cochlear synapses, and overexcitation of hair cells due to high levels of stimulation produces excess levels of glutamate. Excessive glutamate forces ion channels to stay open on the dendrite of the auditory nerve fiber, and the high concentration of calcium ions that enter through these channels can result in cell death. Activation of the HPA axis by stress is also known to raise glutamate levels in specific brain regions. It could potentially induce excitotoxicity if glutamate concentrations are high enough in the cochlea.
The effect of stress on the body’s immune system can also impact hearing function. Cytokines are proteins released by immune cells and are involved in modulating inflammatory responses throughout the body. Some cytokines cause inflammation while others reduce inflammation, which overall can affect cellular function and neural plasticity. Tumor necrosis factor (TNF)-alpha is an inflammatory cytokine that is known to increase in the cochlea after noise-induced hearing loss. One study found that increased TNF-alpha levels measured from the blood were correlated with stress levels and tinnitus severity in tinnitus sufferers.17 Another study showed the opposite effect for an anti-inflammatory cytokine, Interleukin-10, which was lower in older adults with tinnitus compared to those without tinnitus.18 These studies support the view that stress modulation of immune function is implicated in the pathogenesis of tinnitus and could result from the damaging effects of cytokine groups on cochlear health.
A final way15 stress could impact cochlear function concerns cochlear blood supply and the maintenance of the endocochlear potential. Prolonged activation of the sympathetic nervous system elevates blood pressure and leads to vasoconstriction, which could damage the delicate vasculature of the cochlea. Dysfunction of the immune system due to chronic stress could also affect the ability to repair cochlear cells affected by acoustic damage. Because immune cells are found in the stria vascularis, their dysfunction could have additional adverse effects on hair cell health.
Stress effects on central auditory processing
Beyond the cochlea, stress affects the central nervous system in several ways and could contribute to the onset of tinnitus and hyperacusis. For example, tinnitus is associated with altered activity in the limbic system,19 a network of brain regions implicated in emotional regulation and memory involving the hippocampus, hypothalamus, and amygdala. The limbic system strongly interacts with the HPA axis, and chronic stress affects neural plasticity in these brain structures.20 Researchers therefore speculate that tinnitus arises from aberrant neural plasticity because of stress effects on limbic–HPA interactions.21 In support of this view, a 2021 study found increased excitation and reduced inhibition in the hippocampus of rats who developed behavioural signs of tinnitus after chronic stress.22 In this case, stress was induced by restraining the animals, not by noise exposure.
Stress may also directly affect neural plasticity within the central auditory system. One recent animal study proposed a pathway by which hyperacusis develops from the effects of chronic stress on the auditory cortex.23 Researchers chronically treated rats with corticosterone, the rat “version” of cortisol, and subsequently measured the rats’ hearing function and neural responses. Long-term corticosterone exposure led to typical indicators of hyperacusis, such as decreased sound level tolerance and sound avoidance. Interestingly, hearing function measured through DPOAEs and ABRs was normal. However, auditory cortex responses were larger in stressed rats, consistent with evidence of neural hyperactivity, and the number of glucocorticoid receptors (the receptor to which corticosterone binds) also increased in the auditory cortex. The researchers speculated that increased glucocorticoid receptors could generate hyperactivity in the auditory cortex, but corticosterone exposure may have also affected inhibitory neurotransmitters that keep activity levels normal.
Stress after sound exposure
A final connection between stress and tinnitus or hyperacusis may depend on how a person psychologically evaluates a noise exposure. Some exposures are more stressful than others, and an open question is whether low-stress exposures are less harmful for the auditory system. In one instance, Lindgren and Axelsson24 measured temporary threshold shifts (TTS) in 10 teenagers after they were exposed to popular music at 106 dBA, as well as a noise stimulus that was matched to the music stimulus in overall level energy across octave-band frequencies.† In some participants, TTS was lower for the music compared to the noise stimulus, suggesting that factors outside of acoustic stimulation (such as stress) affected thresholds. In another study, participants were exposed to octave-band filtered noise at 90 dB for 30 minutes. Some participants were told that the exposure was a “reward” for performance on a task, while others were told that it was a “punishment.” TTS was greater for the punishment group compared to the reward group, even though the exposures were identical.25
One way to interpret these findings is that lower-stress exposures (music or noise framed as a "reward") inflict less damage to the cochlea. Tinnitus and hyperacusis were not measured, but these conditions would be less likely to develop with lower cochlear damage. A caveat of these TTS studies was that threshold shifts were calculated using audiometry. The effect of stress on perceptual decision making could account for the findings, rather than the effects of noise exposure on cochlear function itself. A person with higher stress may behave conservatively, responding to the presence of tones only when there is high certainty. These decision-making differences might appear as TTS when auditory thresholds are obtained behaviourally.
Conclusions
The effect of stress on the auditory periphery and central nervous system creates several conditions for the development of tinnitus and hyperacusis. Still, there is a considerable lack of research that either confirms or rules out these many explanations. While researchers work to solve these challenges, clinicians can play a role by understanding stress-reducing strategies and paying careful attention to patients’ stress levels. Interventions designed to combat stress and tinnitus, such as mindfulness meditation26 and cognitive-behavioural therapy,27 may be important complements to routine audiological care.
† It would be difficult to justify TTS studies today because these noise exposures can cause permanent loss of cochlear synapses and nerve fibers
References
- Betz, L. T., Mühlberger, A., Langguth, B., & Schecklmann, M. (2017). Stress reactivity in chronic tinnitus. Scientific reports, 7(1), 41521.
- Probst, T., Pryss, R., Langguth, B., & Schlee, W. (2016). Emotional states as mediators between tinnitus loudness and tinnitus distress in daily life: Results from the “TrackYourTinnitus” application. Scientific reports, 6(1), 20382.
- Gans, J. (2016). Mindfulness based tinnitus stress reduction: Unraveling the Gordian Knot of tinnitus. Canadian Audiologist, 3(1).
- Curtis J (1841) Tinnitus aurium. Lancet 36:828–829.
- Baigi, A., Oden, A., Almlid-Larsen, V., Barrenäs, M. L., & Holgers, K. M. (2011). Tinnitus in the general population with a focus on noise and stress: a public health study. Ear and hearing, 32(6), 787-789.
- Russell, G., & Lightman, S. (2019). The human stress response. Nature reviews endocrinology, 15(9), 525-534.
- Chatzaki, E., & Pothoulakis, C. (2019). Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. Journal of Clinical Medicine, 8(10), 1669.
- Yoshida, N., Kristiansen, A., & Liberman, M. C. (1999). Heat stress and protection from permanent acoustic injury in mice. Journal of Neuroscience, 19(22), 10116-10124.
- Wang, Y., & Liberman, M. C. (2002). Restraint stress and protection from acoustic injury in mice. Hearing research, 165(1-2), 96-102.
- Marin, M. F., Lord, C., Andrews, J., Juster, R. P., Sindi, S., Arsenault-Lapierre, G., ... & Lupien, S. J. (2011). Chronic stress, cognitive functioning and mental health. Neurobiology of learning and memory, 96(4), 583-595.
- Bisharat, G., Kaganovski, E., Sapir, H., Temnogorod, A., Levy, T., & Resnik, J. (2025). Repeated stress gradually impairs auditory processing and perception. PLoS Biology, 23(2), e3003012.
- Hébert, S., & Lupien, S. J. (2007). The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neuroscience letters, 411(2), 138-142.
- Hébert, S., & Lupien, S. J. (2009). Salivary cortisol levels, subjective stress, and tinnitus intensity in tinnitus sufferers during noise exposure in the laboratory. International journal of hygiene and environmental health, 212(1), 37-44.
- Alsalman, O. A., Tucker, D., & Vanneste, S. (2016). Salivary stress-related responses in tinnitus: A preliminary study in young male subjects with tinnitus. Frontiers in Neuroscience, 10, 338.
- Szczepek, A. J., & Mazurek, B. (2021). Neurobiology of stress-induced tinnitus. In The behavioral neuroscience of tinnitus (pp. 327-347). Cham: Springer International Publishing.
- Pujol, R., Puel, J. L., D'aldin, C. G., & Eybalin, M. (1993). Pathophysiology of the glutamatergic synapses in the cochlea. Acta oto-laryngologica, 113(3), 330-334.
- Szczepek, A. J., Haupt, H., Klapp, B. F., Olze, H., & Mazurek, B. (2014). Biological correlates of tinnitus-related distress: an exploratory study. Hearing research, 318, 23-30.
- Haider, H. F., Ribeiro, S. F., Martins, C., Ribeiro, D., Trigueiros, N., Szczepek, A. J., ... & Borrego, L. M. (2020). Tinnitus, hearing loss and inflammatory processes in an older Portuguese population. International Journal of Audiology, 59(5), 323-332.
- Lockwood, A. H., Salvi, R. J., Coad, M. L., Towsley, M. L., Wack, D. S., & Murphy, B. W. (1998). The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology, 50(1), 114-120.
- Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in neuro-psychopharmacology and biological psychiatry, 29(8), 1201-1213.
- Patil, J. D., Alrashid, M. A., Eltabbakh, A., & Fredericks, S. (2023). The association between stress, emotional states, and tinnitus: a mini-review. Frontiers in aging neuroscience, 15, 1131979.
- Kim, M. J., Park, S. Y., Park, J. M., Yu, H. J., Park, I., & Park, S. N. (2021). Evidence of tinnitus development due to stress: an experimental study in rats. The Laryngoscope, 131(10), 2332-2340.
- Manohar, S., Chen, G. D., Li, L., Liu, X., & Salvi, R. (2023). Chronic stress induced loudness hyperacusis, sound avoidance and auditory cortex hyperactivity. Hearing Research, 431, 108726.
- Lindgren, F., & Axelsson, A. (1983). Temporary threshold shift after exposure to noise and music of equal energy. Ear and Hearing, 4(4), 197-201.
- Hörmann, H., Mainka, G., & Gummlich, H. (1970). Psychische und physische Reaktionen auf Geräusch verschiedener subjektiver Wertigkeit. Psychologische Forschung, 33(4), 289-309.
- Rademaker, M. M., Stegeman, I., Ho-Kang-You, K. E., Stokroos, R. J., & Smit, A. L. (2019). The effect of mindfulness-based interventions on tinnitus distress. A systematic review. Frontiers in neurology, 10, 1135.
- Fuller, T., Cima, R., Langguth, B., Mazurek, B., Vlaeyen, J. W., & Hoare, D. J. (2020). Cognitive behavioural therapy for tinnitus. The Cochrane database of systematic reviews, 2020(1), CD012614.


