Are COVID-19 “Brain Fog” Symptoms and an Auditory Processing Disorder Related?
Editor’s Note: This article originally appeared in the September 2021 edition of The Hearing Review and appears here courtesy of the publisher. All rights reserved.
When a COVID-19 survivor reports that they have been diagnosed with brain fog or mild cognitive impairment (BF/MCI), or these terms appear in a medical report, hearing care professionals should be aware that many of the BF/MCI symptoms are very similar to those seen in patients with (central) auditory processing disorder. This article reviews the research on this subject and provides recommendations.
Many people have struggled with the aftereffects of COVID-19 for more than 3 weeks or longer after diagnosis.1-3 These side effects include (but are not limited to) fatigue, mild cognitive issues, and low tolerance to mental activity. They also can reoccur at any time with no warning.4
Individuals with these persistent symptoms have been labeled “long-haulers” in the press and social media, most often exhibiting symptoms of mild cognitive impairment (MCI).2,5 However, another term started to appear in professional literature and on social media that described the symptoms of MCI as “brain fog” (BF).
Because this pandemic was like no other that we have seen in over a century, the after-effects of the virus were initially thought to be short term (2-6 weeks), like most other viruses. However, as time went on, this sub-group of survivors (long-haulers) continued to have serious medical issues related to the virus well beyond 2 months.
The Indiana University School of Medicine (IUSM) published a survey of over 1,500 COVID-19 survivors,6 and the authors identified 50 symptoms that included: #1 fatigue (n = 1,567), #4 difficulty concentrating or focusing (n = 924), and #9 memory problems (n = 714). Hearing loss was not listed.
Interestingly, persons with hearing loss usually have the same auditory behaviors and communication complaints that are listed in Table 1a (see also Definitions). It is only when an audiologist conducts a comprehensive evaluation of the peripheral auditory system that a more specific diagnosis can be reached.
Brain Fog: What We Know
A search of the PubMed database (National Library of Science, 2021) identified the first use of the term [brain] “fog” in 2006 when researchers were referencing chemotherapy drug side effects (ie, confusion, memory impairment, etc).7 Also, the term “clouding of consciousness” was a pre-pandemic term used describe similar symptoms or behaviors.8
BF began being used interchangeably with MCI. MCI symptoms include (but are not limited to) confusion, increased memory problems, lack of mental clarity, poor concentration, and an inability to stay focused.9 Fatigue is also a symptom.5 A closer look at the reported BF symptoms reveals that many of these symptoms may be associated with another medically recognized diagnosis: a (central) auditory processing disorder or (C)APD.10 In reviewing the literature that describes the symptoms associated with BF/MCI, it becomes clear that these reported behaviors are not unique because they also appear under other diagnostic labels (see Definitions).
BF is a non-medical term, so there is no direct ICD-10-CM code for it. A “catch-all” code (R41.9) could be used in relation to BF (ie, “Unspecified symptoms and signs involving cognitive functions and awareness”).11 MCI has its own ICD-10-CM code (G31.84)12, as does (C)APD (H93.25).13
Definitions
Several definitions appear in the professional literature that allude to BF. (Note that #2 and #3 below refer to hearing/speech recognition difficulties in the presence of a normal pure-tone audiogram.14)
- Central Auditory Processing Disorder (also referred to as an Auditory Processing Disorder). (C)APD can be defined as a deficit in the auditory areas of the brain resulting in the disruption of processing information specific to the auditory modality.15 It may be exacerbated in unfavorable acoustic environments, and is therefore often associated with difficulties in listening, speech understanding, language development, and learning. It should be noted that similar behaviors of any comorbidities must be considered (eg, Attention Deficit-Hyperactivity Disorder).16
- Obscure Auditory Dysfunction (OAD). An OAD is a clinical presentation whereby the patient experiences difficulty understanding speech in the presence of background noise when there is no measurable hearing loss or other obvious cause. The term was coined in 1989, more than 30 years before the pandemic.17 Details about OAD can be found in Saunders et al.18
- Hidden Hearing Loss (HHL). Musiek et al19 described HHL as difficulty understanding speech in noise despite normal audiograms. Cochlear synaptopathy—the loss of afferent fiber communication at the inner hair cell ribbon synapse—has been proposed as a cause.20
- Adverse Drug Reaction (ADR). An ADR—more commonly referred to as a drug “side effect”—is an unwanted or harmful reaction experienced following the administration of a drug or combination of drugs under normal conditions of use.21
The drug manufacturer and several reliable websites can be searched by both professionals and consumers for the side effects of medication(s) that could be contributing to communication complaints, difficulty attending (staying focused), short-term memory problems, and/or word finding problems. These symptoms are often comorbid with (C)APD or BF/MCI.22-24 Hearing care professionals and pharmacists can work together to establish timelines that might provide the missing piece to the diagnostic puzzle. - An undiagnosed learning disability. Many learning-disabled adults exhibit symptoms that were never formally evaluated, diagnosed, and treated, and have instead developed successful learning and communication strategies over their lifetimes. However, the COVID-19 virus and its effects on communication may have brought about unique strains on thee preexisting neurological condition and/or successful coping strategies of these individuals. Thus, new communication problems may have surfaced under the guise of any of the aforementioned diagnoses.
In fact, in the presence of unremarkable findings on a basic audiological evaluation—including pure-tone thresholds within normal limits and normal otoacoustic emissions (OAEs)—an auditory processing evaluation would be the next recommendation.
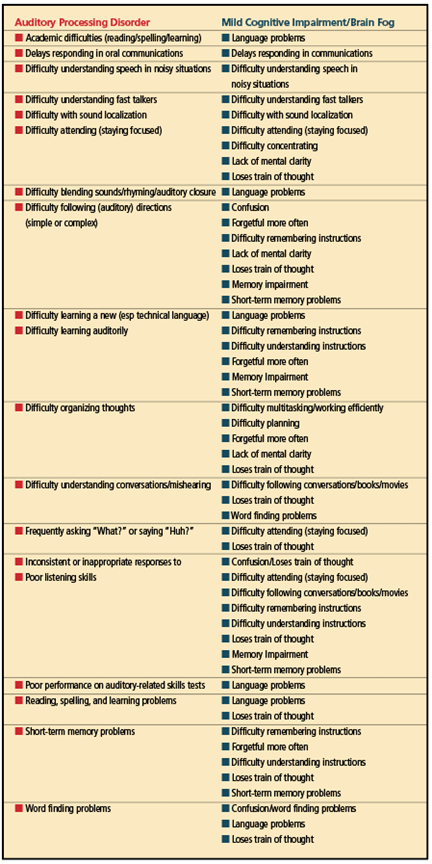
The symptoms of BF/MCI appear in the sidebar in Table 1a (left column) while the behaviors associated with an auditory processing disorder appear in Table 1b (right column). Hopefully, the term “brain fog” will be replaced in the COVID-19 lexicon. Healthcare providers should be made aware that BF might actually stem from an undiagnosed APD or learning disability.
With such a large overlap of symptoms associated with an APD and BF (see Table 2), the medical community should recognize that BF/MCI could possibly be an (central) auditory processing deficit and refer the patient to an audiologist for a (C)APD evaluation that utilizes normative data from evidence-based research.
Brain Shrinkage and Auditory Processing
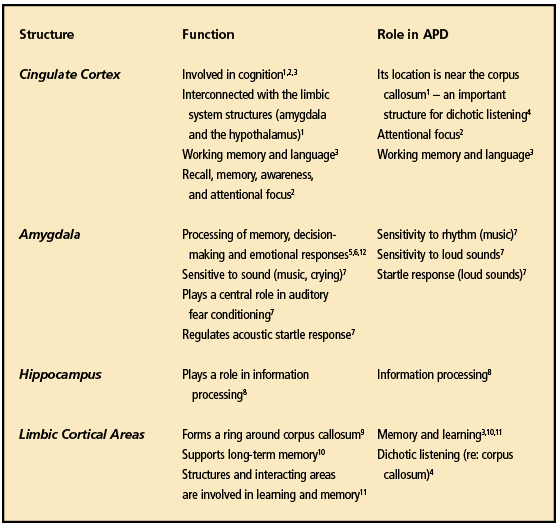
A recent study by Douaud et al25 not only sheds new light on the effects of COVID-19 on specific brain structures, but also the etiology of BF. The findings also strengthen the proposition that BF could possibly be an APD or related to the consequences of an APD especially with known reductions in several brain structures (Table 3).
The researchers analyzed the MRIs of 782 adult subjects who had normal, pre-pandemic MRIs on record at England’s Biobank. A total of 394 of these subjects later contracted COVID-19 (leaving 388 in the control group). Their findings identified a greater loss of gray matter in several areas of the COVID-19 brains, with many of the areas related to auditory processing.
Related article: SARS-CoV-2 is associated with changes in brain structure in UK Biobank (Nature, March 7, 2022)
They noted several anatomical areas that were noticeably smaller when compared to the pre-COVID MRIs. The strongest deleterious effects of COVID-19 were seen predominantly in the left hemisphere and in the right temporal pole—an anatomical landmark that corresponds to the anterior end of the temporal lobe, lying in the middle cranial fossa. The temporal pole has strong connections with the amygdala.26
Could this reduction in size alter the physiology, thus leading to issues in auditory perception? Table 3 lists the anatomical structures of the brain that were found by Douaud et al25 to be reduced and each structure’s role in auditory processing.
Other Neurological Correlates to APD
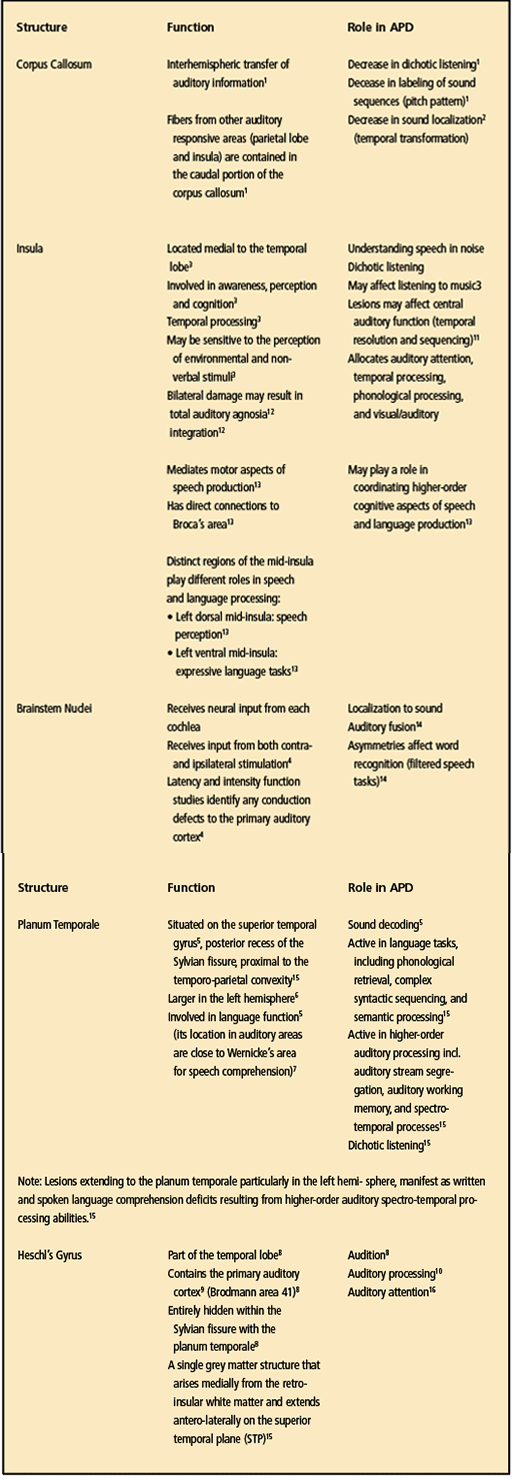
There are other structures in the brain that are located very close to the structures of those identified in the Douaud study.25 These include the corpus callosum, insula, brainstem, planum temporale, and Heschl’s gyrus, (for a summary of these structures and how they relate to APD, see Table 4) Also, these structures may or may not have been affected by the COVID-19 virus; however, we have to assume that the possibility exists that they have been affected given their proximity to the other structures that incurred reductions in gray matter.
Working on that assumption, audiologists will need to select the proven APD tests that are sensitive to these brain structures. Three central auditory test procedures—gap detection, pattern perception, and dichotic listening—show documented value in defining deficits of the central auditory nervous system (CANS) despite the presence of normal pure-tone thresholds.19
It is also important that audiologists know and fully understand the role that these structures have when evaluating a patient suspected of having an APD (personal correspondence, Frank Musiek, PhD, November 2021).
Recovery From COVID-19
A self-organized group of COVID-19 “long-haul” patients—who also happened to be researchers in related science fields—developed and analyzed a patient-led research survey around the COVID-19 experience and their post-recovery issues.27 The research team analyzed 640 survey respondents. Approximately 70% (n = 448) of the respondents reported “brain fog”/concentration challenges. The average length of time of being symptomatic was 27 days for about 10% the respondents. Unrecovered respondents experienced symptoms for an average of 40 days, with a large proportion experiencing symptoms for 5 to 7 weeks. The chance of a full recovery by Day 50 was less than 20%.
Although all patients reported experiencing a full recovery after 90 days (except for patients with pre-existing asthma), 70% experienced fluctuations in their symptoms.
Pavli et al3 reviewed the research evidence on what they labeled as “Post-COVID Syndrome.” Fatigue was the most common symptom reported in up to 72% of post-COVID studies that they reviewed. They noted that mental problems could affect up to 26% of patients. The authors also found that up to 35% of patients with COVID-19 had symptoms that persisted beyond 3 weeks (but with a good prognosis for recovery and no further complications or fatal outcomes).
APD Case History Guidelines
According to a recent international study by Davis et al,28 the post-COVID incidence of BF, hearing loss, tinnitus, and/or vertigo increases sharply within 1 and 2 months after the diagnosis (n = 3672). There were no ENT issues in the first 2 weeks after the diagnosis. Cognitive dysfunction was found in all age groups and was the third most common debilitating issue (n = 1274) experienced by these patients with the #1 most-common issue being fatigue (n = 2652) followed by respiratory problems (n = 2242).
The importance of establishing timelines is critical.23,24 Hearing loss is likely to occur at the peak of infection because the blood-labyrinth barrier is damaged/destroyed during this period.29 Establishing a better relationship with the patient’s pharmacist will be part of the success in determining if the symptoms are virus-related.
The traditional case history questions might not provide sufficient information with a COVID-19 patient. In fact, it would be beneficial to develop a separate COVID-19 Survivor Case History form given the current “unknowns” about the virus. In particular, audiologists need to determine if the cognitive complaints might be a new diagnosis or a change/deterioration/exacerbation of a pre-existing condition/comorbidity.30 Also, the communication complaints could be related to the patient’s COVID-19 medication history or from a change in the patient’s current drug regimen. This underscores the importance of establishing a communication channel with the patient’s pharmacist.
Related Article: Six Points of Audiological Consensus on Central Auditory Processing Disorders (CAPD)
It should also be apparent that the use of a self-assessment tools, such as the Hearing Handicap Inventory for Adults31 or the Hearing Handicap Inventory for the Elderly,32 can be very effective for learning more about the patient before testing begins. Because of the unknown cognitive impairments from COVID-19, the patient should have a close family member or friend accompany them to confirm the answers to the medical history.33,34
The American Academy of Audiology (AAA) provides an excellent guideline for taking a case history when an auditory processing disorder is suspected.35
APD Testing and Diagnosis
The diagnosis of (C)APD should be made on the basis of a carefully selected battery of sensitive and specific behavioral tests and electrophysiologic procedures, supplemented by observation and detailed case history.35
It is beyond the scope of this article to go into details as to which auditory processing tests to perform. However, Musiek et al19 have recommended the following in addition to a comprehensive audiological evaluation:
- Middle latency response evoked potentials
- Speech-in-noise testing
- Tests for localization
- Gap detection
- Pattern perception (frequency and duration), and
- Dichotic listening tests
Audiologists interested in pursuing this series of tests for COVID-19 survivors should familiarize themselves with the various procedures and interpretations; how to report the findings; as well as recommendations for ongoing management.
This area needs additional research before changes/additions to existing (C)APD test protocols can be developed for patients with a history of COVID-19.
Guarded Prognosis
Monitoring the long-term trajectory and neurocognitive outcomes of COVID-19 patients is critical. These outcomes include anxiety, depression, and post-traumatic stress (PTS) disorder symptoms and neuropsychiatric disorders. These disorders could cause a predisposition to neurodegeneration.36
For audiologists this is a critical notation because cognitive issues can mimic hearing loss (eg, focusing, paying attention, asking “What?” when spoken to, and memory issues). Therefore, although new patients might complain of hearing loss, the behavioral audiometric test results may not show any peripheral hearing loss. Objective tests could also be normal.
Because there are still many unknowns about the virus (especially the newer Delta and Omicron variants), the patient’s primary healthcare provider should refer the patient to an audiologist for a comprehensive audiological and (C)APD evaluation.
The Importance of an Interprofessional Team Approach
The diagnosis and long-term management of COVID-19 patients is not the same as most previously identified viruses (eg, influenza). Over the past 18 months, we have learned that the virus (and its lingering effects on other body systems) requires an interprofessional intervention. This relationship among healthcare providers is to identify any comorbidities that can impact management strategies. A central source for all healthcare providers can be very effective, with all the medical information easily accessible.
Doheny37 identified 11 professions which could be involved in short- and long-term management of COVID-19 patients:
- Cardiology
- Infectious disease specialists
- Nephrology
- Neurology
- Occupational therapy
- Pharmacy
- Primary care physicians
- Pulmonary medicine
- Physical therapy
- Radiology
- Social work
Unfortunately, she did not identify audiology or speech-language pathology as specialties that should be involved in patient management.
Future Directions: Cognitive Screening in Audiology Practice
Hearing care professionals understand that a patient’s cognitive issues can impact their ability to process speech (especially in noisy environments) which, in turn, could affect response time and recall/memory.
A cognitive (non-diagnostic) screening test can be performed by a licensed audiologist for the purpose of confirming a medical concern, thus leading to a referral to another professional for further evaluation.38,39 (For more details, see Principle 2, Rules 2a – 2e, and Principles 3 and 4 of the AAA Code of Ethics.40)
Additional training would be needed for audiologists wishing to perform a relatively quick screening measure that would include tests for memory and attention. For example, the Montreal Cognitive Assessment (MoCA) is a brief, 30-question test that can detect early cognitive impairments thus resulting in a referral for additional psychological services.41
Related Article: Issues in Cognitive Screenings by Audiologists
Davis42 reported that a non-verbal cognitive screening test that utilizes computerized, self-administered programs (ie, Cognivue Thrive, Cogstate, and NeuroTrax) removes the auditory component from the equation and isolates cognition. Other screening tests include the General Practitioner Assessment of Cognition (GPCOG) and the Mini-Mental State Examination (MMSE).43
A detailed review of other screening tests for cognitive impairment can be found in Cullen et al.44 A more recent overview of cognitive screening tests for hearing care professionals was provided by guest editors Douglas Beck, AuD, and Michael Harvey, PhD, ABPP, in the special edition of the January 2021 Hearing Review.39
Diagnoses Code for Third-party Payors
The following ICD-10-CM Diagnosis Codes might be ascribed to a COVID-19 survivor depending on test results of an interprofessional team:
- Mild Cognitive Impairment (MCI): ICD-10-CM Code G31.84.12
- (Central) Auditory Processing Disorder: ICD-10-CM Code H93.25.13
- Brain Fog (BF): ICD-10-CM Code R41.9 (Unspecified symptoms and signs involving cognitive functions and awareness).11
Of course, there is no guarantee of payment for services depending on the terms of the patient’s contract and the provider’s participation.
Summary
COVID-19 has opened a Pandora’s box of issues relative to the lingering ailments experienced by “long-haulers” with Post-COVID Syndrome. Comorbidities may exacerbate complaints. An audiologist is well qualified to assess a patient both subjectively and objectively based on the patient’s communication complaint(s) or concerns by family members and friends.
When a COVID-19 survivor reports that they have been diagnosed with BF/MCI or these terms appear in a medical report, hearing care professionals should review the behaviors that appear in Table 1a. Audiologists have seen a subset of these behaviors in children and adults suspected of having an APD (note the behaviors that appear in Table 1b).
A further comparison of both diagnoses reveals 16 symptoms MCI/BF and an APD that are quite similar, as shown in Table 2. Table 3 shows the brain areas that were identified by Douaud et al25 as having less gray matter post-COVID. Therefore, can we assume that, if these cortical areas have less gray matter post-COVID-19, that the surrounding regions could also show less gray matter? In the author’s opinion, reanalysis and more research concerning the data from the Douaud study25 focusing on the insula, brainstem nuclei, planum temporale, and Heschl’s gyrus should answer this question.
However, when the term BF is being used in a report (or the patient reports having BF), it should immediately raise suspicion that what they are referring to is possibly an APD. Testing to rule out APD should be scheduled as soon as possible.
The non-medical term “brain fog” has been used by patients to describe fatigue-related, memory/recall-related, focusing/concentrating, and having word-finding problems. Audiologists have heard these complaints regularly by patients who have been diagnosed with an auditory processing disorder.
But is it truly “brain fog” or a mild cognitive impairment? Evidence-based research has already identified the same symptoms that audiologists refer to as an auditory processing disorder (APD). Also, there are additional labels that could be ascribed to someone who presents with BF/MCI:
- Obscure Auditory Dysfunction (OAD)
- Hidden Hearing Loss (HHL)
- Adverse Drug Reactions (ADR)
- Undiagnosed Learning Disability
In my opinion, the removal of the use of the term “brain fog” from the COVID-19 vernacular by healthcare providers should be addressed by the audiology associations. After audiological and (central) auditory processing testing, the diagnosis might be written as “an auditory processing disorder secondary to COVID-19 Syndrome” (another diagnostic not recognized by the medical profession).
Of course, more evidence-based research is needed at this time as we learn more about the long-term effects of COVID-19 on the auditory system.
Recommendations
- The AAA, the American Speech-Language-Hearing Association (ASHA), and the Academy of Doctors of Audiology (ADA) should review these issues and draft a joint statement to the American Medical Association (AMA), American Psychological Association (APA), and other allied professions encouraging their members to stop using the generic, non-medical, non-scientific term “brain fog” in their vernacular. Instead, they should refer to these patients as having COVID-19 Syndrome and recommend patients be referred for an auditory processing evaluation to inform treatment management (see Baig 202045).
- Audiologists should reach out to their local healthcare providers and educate them about “brain fog” symptoms and the symptoms associated with an APD detailed in this article.
- A closer relationship with the patient’s pharmacist will be very helpful in establishing medication timelines, especially when comorbidities have been identified.
- Objective (electrophysiological) testing is an important component to the differential diagnostic test battery. Otoacoustic emissions (OAEs, transient and distortion product), auditory brainstem response (ABR) testing, and the Auditory Middle Latency Response Test (AMLR) should be considered with the rationale being that the primary auditory cortex is an essential region of the CANS in auditory processing of speech and non-speech signals.
- Subjective testing (especially word recognition tests in noise) should also include gap detection, pattern perception, and dichotic listening tests. These auditory processing tests show documented value in defining deficits of the CANS.35 A re-evaluation of the test battery used for (C)APD testing may need to be revisited for these patients.
- Recommendations for management (including the use of hearing assistance technologies) should be addressed on a case-by-case basis.
- Hearing care professionals should learn how to administer a cognitive screening test and make any additional recommendations for intervention and management.
- Hearing care practice owners should reach out to local media to inform their community that the term “brain fog” could possibly be an auditory processing disorder which can be evaluated using audiological and central auditory processing tests that are supported by evidence-based research—and that management strategies exist.
- A specific testing protocol should be developed for COVID-19 patients.
Acknowledgements
The author gratefully acknowledges the suggestions and recommendations made to this manuscript by the distinguished researcher in auditory processing, Frank Musiek, PhD, at the University of Arizona. Also, the suggestions made by Larry Engelmann, AuD, (as an independent reader) are also gratefully appreciated. The opinions expressed in this manuscript are solely those of the author and not the publisher or the aforementioned independent contributors.
Correspondence can be addressed to HR or Dr DiSogra at: bobdisogra@gmail.com
Citation for this article: DiSogra RM. Are COVID-19 “brain fog” symptoms and Auditory processing disorder related? Hearing Review. 2022;29(3):28-38.
References
- Baig AM. Deleterious outcomes in long-hauler COVID-19: The effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci. 2020;11(24):4017–4020
- Cleveland Clinic Health Essential website. What it Means to Be a Coronavirus “Long-hauler.” https://health.clevelandclinic.org/what-it-means-to-be-a-coronavirus-long-hauler/. Published January 28, 2021. Accessed May 16, 2021.
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: Incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52(6):575-581.
- National Institutes of Health (NIH) website. Citizen Scientists Take on the Challenge of Long-haul COVID-19. https://www.nih.gov/citizen-scientists-take-challenge-long-haul-covid-19. Published September 3, 2020. Accessed May 1, 2021.
- Centers for Disease Control and Prevention (CDC) website. Subjective Cognitive Decline – A Public Health Issue. https://www.cdc.gov/aging/data/subjective-cognitive-decline-brief.html. Published February 27, 2019. Accessed May 16, 2021.
- Lambert NJ, Survivor Corps. COVID-19 “Long hauler” Symptoms Survey Report. Indiana Univ School Med; 2020:1-13.
- Raffa RB, Duong PV, Finney J, et al. Is ‘chemo-fog’/’chemo-brain’ caused by cancer chemotherapy? J Clin Pharm Ther. 2006;31(2):129-38
- Schildkrout B. Unmasking Psychological Symptoms: How Therapists Can Learn to Recognize the Psychological Presentation of Medical Disorders. Hoboken, NJ:Wiley Publishing; 2011; 183–184.
- Healthline website. What to Know About COVID-19 and Brain Fog. https://www.healthline.com/health/covid-brain-fog. Published March 17, 2021. Accessed May 9, 2021
- American Speech-Language-Hearing Association (ASHA) website. Central Auditory Processing Disorder. https://www.asha.org/practice-portal/clinical-topics/central-auditory-processing-disorder/.
- ICD10Data website. Unspecified Symptoms and Signs Involving Cognitive Functions and Awareness. Code R41.9 (2021). Accessed May 2, 2021
- ICD10Data. Mild cognitive impairment, so stated. Code G31.84 (2021). Accessed May 2, 2021
- ICD10Data. Central auditory processing disorder. Code H93.25 (2021). Accessed May 2, 2021
- Chermak G. 20Q: CAPD – Diagnosis and Intervention. AudiologyOnline. https://www.audiologyonline.com/articles/20q-capd-diagnosis-and-intervention-17875. Published August 8, 2016.
- Jerger J, Musiek F. Report of the consensus conference on the diagnosis of auditory processing disorders in school-aged children. J Amer Acad Audiol. 2000;11(9):467-474
- Chermak GD, Bamiou D-E, Iliadou V, Musiek FE. Practical guidelines to minimise language and cognitive confounds in the diagnosis of CAPD: A brief tutorial. Int J Audiol. 2017;56(7):499-506.
- Saunders GH, Haggard MP. The clinical assessment of obscure auditory dysfunction – 1. Auditory and psychological factors. Ear Hear.1989;10(3):200-208.
- Saunders GH, Field DL, Haggard MP. A clinical test battery for obscure auditory dysfunction (OAD): Development, selection and use of tests. Br J Audiol. 1992;26(1):33-42.
- Musiek FE, Chermak GD, Bamiou D-E, Shinn J. CAPD: the most common ‘hidden hearing loss.’ ASHA Leader. https://leader.pubs.asha.org/doi/10.1044/leader.FMP.23032018.6. Published March 1, 2018.
- Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017;349:138-147
- The International Union of Basic and Clinical Pharmacology (IUPHAR) website. Pharmacology Education Project. Adverse Drug Reactions. www.pharmacologyeducation.org/clinical-pharmacology/adverse-drug-reactions. Accessed May 10, 2021
- DiSogra RM. Drug side-effects on audiological and vestibular testing. ENT & Audiology News. https://www.entandaudiologynews.com/features/audiology-features/post/drug-side-effects-on-audiological-and-vestibular-testing. Published November 1, 2016.
- DiSogra RM. Drug side effects on hearing and balance testing. Hear J. 2018;71(6):10-14.
- DiSogra RM. The impact of pharmaceutical side effects on audiological and vestibular measurements. Sem Hear. 2019;40(2): 97-103.
- Douaud G, Lee S, Alfaro-Almagro F, et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv [Preprint].June 15, 2021. DOI: https://doi.org/10.1101/2021.06.11.21258690.
- Luijkx T. Temporal Pole. https://radiopaedia.org/articles/temporal-pole?lang=us. Published March 14, 2015. Accessed July 16, 2021.
- Patient-Led Research Collaborative. Report: What does COVID-19 recovery actually look like? https://patientresearchcovid19.com/research/report-1/#Recovery_Timecourse. Published May 11, 2020. Accessed May 1, 2021.
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38(101019).
- Satar B. Criteria for establishing an association between Covid-19 and hearing loss. Amer J Otolaryngol. 2020;41(6):102658.
- Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2:1069-1076.
- Newman CW, Weinstein BE, Jacobson GP, Hug GA. The Hearing Handicap Inventory for adults: Psychometric adequacy and audiometric correlates. Ear Hear.1990;11(6):430-433
- Ventry IM, Weinstein B. The hearing handicap inventory for the elderly: A new tool. Ear Hear 1982;3(3):128-134
- DiSogra RM. COVID-19 and the hearing care professional: What we know so far. Hearing Review. 2021;28(9):18-21.
- DiSogra RM. Labeling COVID-19’s long-term effects—What’s the diagnosis? AudiolToday. https://www.audiology.org/news-and-publications/audiology-today/articles/online-feature-labeling-covid-19s-long-term-effects-whats-the-diagnosis/.
- American Academy of Audiology (AAA). Clinical Practice Guidelines. Guidelines for the diagnosis, treatment and management of children and adults with central auditory processing disorder.Published August 2010.
- Pero A, Ng S, Cai D. COVID-19: A perspective from clinical neurology and neuroscience. The Neuroscientist. 2020;26(5-6):387–391.
- Doheny K. Long COVID: More Clues Coming, but No ‘Aha’ Moments Yet. Medscape. https://www.medscape.com/viewarticle/962761. Published November 11, 2021.
- Beck DL, Weinstein BE, Harvey M. Issues in cognitive screenings by audiologists. Hearing Review. 2016;23(2):36-40.
- Beck DL, Harvey M. Issues in cognition, audiology, and amplification. Hearing Review. 2021;28(1):28-33
- American Academy of Audiology (AAA) website. Code of Ethics. https://www.audiology.org/clinical-resources/code-of-ethics/. Published October 2019. Accessed May 25, 2021.
- MoCA Cognitive Assessment Test website. www.mocatest.org.
- Davis J. Cognitive screening in audiology: Considerations for nonverbal instructions. Hear J. 2021;74(5):34-35.
- Nalley C. Practice benefits of integrating cognitive screening services. Hear J. 2021;74(4):26-28.
- Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;78(8.
- Baig AM. Chronic COVID Syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93(5):2555-2556.
References for Table 1a
- Alzheimer’s Association website. Mild Cognitive Impairment. www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment. Accessed May 1, 2020.
- American Academy of Audiology (AAA). Clinical Practice Guidelines. Guidelines for the diagnosis, treatment and management of children and adults with central auditory processing disorder. https://audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%208-2010.pdf_539952af956c79.73897613.pdf. Published August 2010. Pages 7-9.
- American Speech-Language-Hearing Association (ASHA) website. Central Auditory Processing Disorder. https://www.asha.org/practice-portal/clinical-topics/central-auditory-processing-disorder/.
- Cleveland Clinic website. Mild Cognitive Impairment. https://my.clevelandclinic.org/health/diseases/17990-mild-cognitive-impairment. Published March 18, 2019. Accessed May 1, 2021.
- Centers for Disease Control and Prevention (CDC) website. Subjective Cognitive Decline – A Public Health Issue. https://www.cdc.gov/aging/data/subjective-cognitive-decline-brief.html. Published February 27, 2019. Accessed May 9, 2021.
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML and Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): An integrative classification. Int J Environ Res Public Health.2021;18(5):2621.
- Geffner D. Central auditory processing disorders: definition, description, behaviors, and comorbidities. In: Geffner D, Ross-Swain D. Auditory Processing Disorders – Assessment, Management, and Treatment. 3rd ed. San Diego, CA: Plural Publishing; 2019: 37-68.
- Harvard Health Publishing website. Is that brain fog really adult ADHD? https://www.health.harvard.edu/diseases-and-conditions/is-that-brain-fog-really-adult-adhd. Published November 1, 2018.
- Budsen AE. What is COVID-19 brain fog – and how can you clear it? Harvard Health Blog. https://www.health.harvard.edu/blog/what-is-covid-19-brain-fog-and-how-can-you-clear-it-2021030822076. Published March 8, 2021.
- Healthline website. What to know about COVID-19 and brain fog. https://www.healthline.com/health/covid-brain-fog. Published March 17, 2021. Accessed May 9, 2021.
- Kingstone T, Taylor AK, O’Donnell CA, Atherton H, Blane DN, Chew-Graham CA. Finding the ‘right’ GP: A qualitative study of the experiences of people with long-COVID. BJGP Open. 2020;4(5):bjgpopen20X101143.
- Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221-2230.
- Mayo Clinic website. Mild Cognitive Impairment. www.mayoclinic.org/diseases-conditions/mild-cognitive-impairment/symptoms-causes/syc-20354578.
- Memorial Sloan Kettering Cancer Center website. MSK researchers learn what’s driving ‘brain fog’ in people with COVID-19. https://www.mskcc.org/news/msk-researchers-learn-what-s-driving-brain-fog-people-covid-19#:~:text=One%20of%20the%20dozens%20of,to%20psychosis%20and%20even%20seizures. Published february 8, 2021. Accessed May 9, 2021.
- Prioleau T. Learning from the experiences of COVID-19 survivors: Web-based survey study. JMIR Form Res. 2021;5(5)e23009.
- Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining long COVID: Striking differences across literature, electronic health records, and patient-reported information. medRxiv [Preprint]. 2021. DOI: https://doi.org/10.1101/2021.03.20.21253896.
- Yetman D. What to know about COVID-19 and brain fog. Healthline. Published 2021.
References for Table 1b
- American Academy of Audiology (AAA). Clinical Practice Guidelines. Guidelines for the diagnosis, treatment and management of children and adults with central auditory processing disorder. https://audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%208-2010.pdf_539952af956c79.73897613.pdf. Published August 2010. Pages 7-9.
- American Speech-Language-Hearing Association (ASHA) website. Central Auditory Processing Disorder. https://www.asha.org/practice-portal/clinical-topics/central-auditory-processing-disorder/.
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML and Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): An integrative classification. Int J Environ Res Public Health.2021;18(5):2621.
- Geffner D. Central auditory processing disorders: definition, description, behaviors, and comorbidities. In: Geffner D, Ross-Swain D. Auditory Processing Disorders – Assessment, Management, and Treatment. 3rd ed. San Diego, CA: Plural Publishing; 2019: 37-68.
- Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221-2230.
- Moore DR. Auditory processing disorder (APD): Definition, diagnosis, neural basis, and intervention. Audiol Med. 2006;4(1):4-11.
- National Institute on Deafness and Other Communication Disorders (NIDCD) website. Auditory Processing Disorder. https://www.nidcd.nih.gov/health/auditory-processing-disorder. Published June 7, 2010. Accessed May 9, 2021
- Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining long COVID: Striking differences across literature, electronic health records, and patient-reported information. medRxiv [Preprint]. 2021. DOI: https://doi.org/10.1101/2021.03.20.21253896.
References for Table 3
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (1):279-306.
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12-32.
- Belkhiria C, Vergara RC, San Martín S, et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Frontiers in Aging Neurosci. 2019;11.
- Bamiou D-E, Sisodiya S, Musiek FE, Luxon LM. The role of the interhemispheric pathway in hearing. Brain Res Rev. 2007;56(1):170-182.
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol. 2005;210:343-352.
- Musiek F, Mohanani A, Wierzbinski E, Kilgore G, Hunter J, Marotto J. The non-classical pathway: Too great to be ignored. Hear J. 2011;64(10):6-8.
- Kraus KS, Canlon B. (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res. 2012;288(1-2):34-46.
- Hacking C. Hippocampus. Radiopaedia. https://radiopaedia.org/articles/hippocampus?lang=us. Published December 27, 2021. Accessed July 13, 2021.
- Chanes L, Feldman-Barrett L. Redefining the role of limbic areas in cortical processing. Trends Cog Sci. 2016;20(2):P96-P106.
- MedlinePlus website. Limbic System. https://medlineplus.gov/ency/imagepages/19244.htm. Published February 4, 2020. Accessed July 13, 2021
- Schacter DL, Gilbert DT, Nock MK, Wegner DM. Psychology. 5th ed. New York, NY: Worth Publishers; 2019.
- Li XF, Phillips R, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105:87-100.
References for Table 4
- Musiek FE. Neuroanatomy, neurophysiology, and central auditory assessment. Part III: Corpus callosum and efferent pathways. Ear Hear. 1986;7(6):349-358.
- Bamiou D-E, Sisodiya S, Musiek FE, Luxon LM. The role of the interhemispheric pathway in hearing. Brain Res Rev. 2007;56(1):170-182.
- Musiek F. What about the insula? Pathways. https://hearinghealthmatters.org/pathways/2017/what-about-the-insula/. Published April 5, 2017. Accessed July 16, 2021.
- Schofield BR, Beebe, NL. The efferent auditory system: Central pathways that modulate peripheral input. The Senses: A Comprehensive Reference. 2020;2:501-516.
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29(1):26-49.
- Musiek FE, Reeves AG. Asymmetries of the auditory areas of the cerebrum. J Am Acad Audiol. 1990;1(4):240-245
- Baars BJ, Gage NM. Hearing and speech. In: Baars BJ, Gage NM. Cognition, Brain, and Consciousness. 2nd ed. Amsterdam, Netherlands: Elsevier; 2010; 194-236.
- Deng F. Heschl’s Gyrus. https://radiopaedia.org/articles/heschls-gyrus-1?lang=us. Published November 27, 2021. Accessed July 5, 2021
- Operative Neurosurgery website. Heschl’s Gyrus. https://operativeneurosurgery.com/doku.php?id=heschl_s_gyrus.
- Warrier C, Wong P, Penhune V, et al. Relating structure to function: Heschl’s gyrus and acoustic processing. J Neurosci. 2009; 29(1):61-69.
- Bamiou DE, Musiek FE, Stow I, et al. Auditory temporal processing deficits in patients with insular stroke. Neurol. 2006;67(4).
- Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing: Literature review. Brain Research Reviews. 2003;42(2):143-154.
- Oh A, Duerden EG, Pang EW. The role of the insula in speech and language processing. Brain and Lang. 2014;135:96-103.
- Palva A, Jokinen K. The role of the binaural test in filtered speech audiometry. Acta Otolaryngol. 1975;79;(3-6):310-314.
- Wong BM, Musiek FE. Morphological variance and related taxonomy of the planum temporale. J Hear Sci. 2020;10(4):9-19.
- Hu M, Wang D, Ji X, et al. Neural processes of auditory perception in Heschl’s gyrus for upcoming acoustic stimuli in humans. Hear Res. 2020;388:107895.