Audiology of the Future with Whole Human Genome Sequencing: Part 2
Renowned auditory researcher Dr. Robert Harrison brings us up to date on information and research from the Labs. Appropriately titled “From the Labs to the Clinics”, Bob is involved in laboratory and applied/clinical research, including evoked potential and otoacoustic emission studies and behavioural studies of speech and language development in children with cochlear implants. For a little insight into Bob’s interests outside the lab and the clinic, we invite you to climb aboard Bob’s Garden Railway.
This is a follow-up from my previous column, to further discuss applications of whole genome sequencing (WGS) in audiology. I shared my article with a group of audiology colleagues and trainees and had extensive feedback of ideas, opinions, and suggestions for further reading on this topic. That feedback from audiologists is the basis for the present commentary.
The general reaction to the possible value of WGS in audiology was enthusiastic but tempered with caution about technical issues in sequencing, interpretation of the complex data sets, and overestimating the value of information obtained. There are also commercial issues, such as patent rights, and regulation in private and public healthcare systems, and looming large are ethical issues around informed consent, confidentiality, and data security.
It was recognized that we have many conditions of uncertain etiology in audiology. I would suggest that we have no clear idea of causation for most hearing and balance problems. We have some very broad diagnostic categories such as “sensorineural hearing loss,” “auditory neuropathy SD,” “age-related hearing loss,” “Meniere’s disease,” etc. wherein are hearing/vestibular problems of multiple causes. Even when a very conspicuous event such as noise trauma or viral infection is known, there are other factors (genetic predisposition; co-morbidities) that, if known, can define etiology more accurately. This is one area in which WGS might provide diagnostic refinements that can help in treatment, interventions, and prognosis.
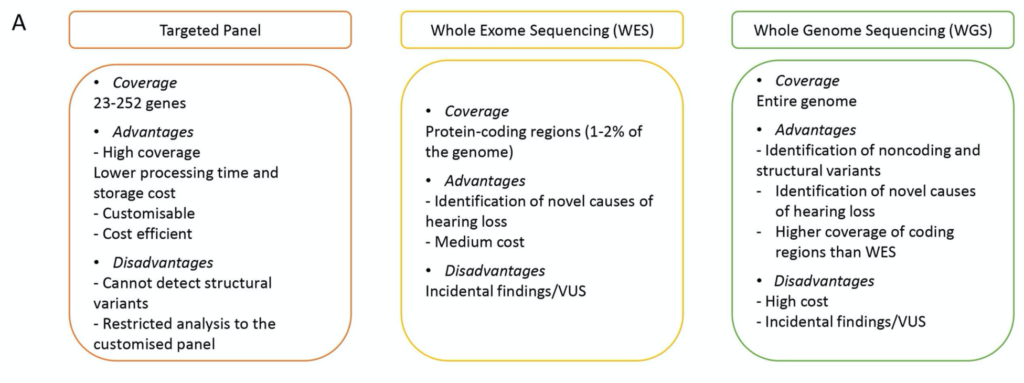
We already have much experience screening for known genetic causes of hearing loss using targeted panels. The figure below compares the potential of the targeted panel with more detailed whole exome and whole genome sequencing. This figure is reproduced from a useful review by Lara Kamal and Karen Avraham titled “What you need to know about recent advances in the genetics of hearing loss in the newborn,” published this year in ENT and Audiology News.1 (Note that Dr. Avraham is a world leader in this field.)
The coverage, advantages and disadvantages of each type of genetic analysis is well summarized. I strongly believe that this newborn genetic screening for the cause of hearing loss will be the first major application for WGS in the audiology field.
There is no doubt that accurate genetic diagnosis can guide the optimal intervention and treatment. For example, it might predict the possible progression of hearing loss and determine the best management and habilitation options. This might include the choice of a hearing aid versus a cochlear implant, signing and/or total communication, etc. One important stage in developing clinically useful strategies is the collection of data that makes the link between genetic diagnosis and the successful course of treatment. When we catalog these connections, genetic information can directly guide the most appropriate interventions.
Turning from “start of life” genetic screening to using WGS in understanding more about “end of life” hearing loss. I draw your attention to a publication titled “Whole-genome sequencing reveals new insights into age-related hearing loss.”2 This is not a clinical paper with practical advice but a very detailed scientific study seeking (as the title suggests) new insights into age-related hearing loss. WGS was carried out on 212 subjects older than 50 years. Sequencing was the first part of a multistep strategy toward the “molecular characterization” of age-related hearing loss. More about this in a future column.
Let me end the present discussion with a summary review of the pros and cons of WGS in audiology. Current or potential advantages include:
- Newborn screening for early detection of genetic causes of hearing loss and early intervention.
- Help in decisions about treatment strategy. Help in the choice or programming of a hearing prosthesis. Help in planning habilitation strategy
- Identify high-risk patients for hearing loss, tinnitus, or vestibular disorders.
- Finding subjects with a genetic disposition for noise-induced hearing loss or susceptibility to ototoxic drugs.
- Determine if hearing loss is likely to be progressive.
- Identification of older subjects where hearing loss is associated with cognitive decline.
- Provide accurate determination of etiology in types of hearing loss that currently are a mixed bag (i.e., a spectrum disorder) such as auditory neuropathy, Meniere’s disease, (central) auditory processing disorder, and SNHL.
Regarding the negatives, the table of challenges below is reproduced from a paper titled: “Whole Genome Sequencing as a Diagnostic Test: Challenges and Opportunities.”3 This is a comprehensive overview of WGS as applied to any clinical disorder.
Challenges
| Cost | Usually Underestimated |
| Technological | Error rate; sequence completeness; inter-instrumental variability; sequencing depth; base-calling algorithms; aligning read algorithms |
| Interpretative | Incidental findings, how to report them to patients and families; how to prioritize variants to predict future effects and use them to counsel patients; impact of ethnicity; lack of sufficient numbers of genetic counselors; physician education. |
| Ethical | Informed consent including pretest and post-test counseling; avoid harm (physical and psychological); disclosure of data linked to behavioral issues or psychiatric disorders; genome sequencing and discrimination; privacy and data security |
| Efficacy | Weak evidence for efficacy of genome sequencing to predict future disease risk in asymptomatic individuals |
| Patents | Infringement on current patents protecting disease-associated mutations. |
The table lists potential challenges in several areas, including technical issues, the interpretation of genetic findings, commercial/legal issues, and ethics. It is regrettable, but these privacy, confidentiality, and data security concerns around genetic testing will likely be the major stumbling block to introducing WGS in healthcare.
Acknowledgements
I had (online) discussions with several audiologists (worldwide) on this topic, and their feedback provided useful ideas and references. In particular, I would like to thank Krishna Priya Vimal Kumar, Inas Alrubaye, Saba Zafar, Chris Radford, Taha Al-Shalash, and Nadia Vas Falcao for their contributions.
References
- Kamal, L. Avraham, K.B. What you need to know about recent advances in genetics of hearing loss in the newborn. ENT & Audiology news. Vol. 31 Issue 6. January/February 2023. Available at: https://www.entandaudiologynews.com/features/audiology-features/post/what-you-need-to-know-about-recent-advances-in-genetics-of-hearing-loss-in-the-newborn
- Vuckovic, D., Mezzavilla, M., Cocca, M. et al. Whole-genome sequencing reveals new insights into age-related hearing loss: cumulative effects, pleiotropy and the role of selection. Eur J Hum Genet 26, 1167–1179 (2018). https://doi.org/10.1038/s41431-018-0126-2
- Caitlin C Chrystoja, Eleftherios P Diamandis. Whole Genome Sequencing as a Diagnostic Test: Challenges and Opportunities, Clinical Chemistry, Volume 60, Issue 5, 1 May 2014, Pages 724–733, https://doi.org/10.1373/clinchem.2013.209213

