Do Infants with Hearing Loss Listen Like Little Adults?
 Rapid developments occur in speech perception over the first year of life. Within hours of birth, normal hearing (NH) infants differentiate their mother’s voice from a stranger’s,1 rhythmic pattern of their native language versus non-native language2, and discriminate speech sounds from around the world.3-5 A decline in NH infants’ abilities to discriminate non-native speech sounds is observed around 6–12 months of age, followed by improved speech discrimination of their native language.4 This rapid perceptual change indicates that early auditory experience is important for the development of speech perception and word recognition. In contrast to our knowledge about infants with NH, little is known about auditory development in infants with hearing loss (HL) and how hearing aid (HA) processed speech may impact development.
Rapid developments occur in speech perception over the first year of life. Within hours of birth, normal hearing (NH) infants differentiate their mother’s voice from a stranger’s,1 rhythmic pattern of their native language versus non-native language2, and discriminate speech sounds from around the world.3-5 A decline in NH infants’ abilities to discriminate non-native speech sounds is observed around 6–12 months of age, followed by improved speech discrimination of their native language.4 This rapid perceptual change indicates that early auditory experience is important for the development of speech perception and word recognition. In contrast to our knowledge about infants with NH, little is known about auditory development in infants with hearing loss (HL) and how hearing aid (HA) processed speech may impact development.
Differences in how NH infants discriminate speech may be explained by their use of temporal envelope (TE; slow amplitude variations) and temporal fine structure (TFS; fast amplitude variations) information.6 We know that vowel discrimination for infants with NH require better spectral resolution than older children and adults.7 For adults with NH and HL, successful speech discrimination in quiet is possible with only TE. The addition of TFS improves speech understanding in noise, although the amount of benefit differs between those with NH and those with HL.8,9 However, little is known about how infants with HL use TE and TFS. Furthermore, consideration should be given to how HA signal processing using nonlinear algorithms (e.g., wide dynamic range compression, frequency lowering, noise reduction) affects these abilities, as nonlinear processing result in modifications either to the TE, the TFS, or both.
To examine the impact of HA signal processing on infant speech discrimination we quantified the amount of TE modification using a well-established metric, Cepstral Correlation (CepCorr10). CepCorr has been used to examine the cumulative effects of signal modification using commercial HAs, and as such does not consider each algorithm individually. Instead, CepCorr considers the overall HA output in the context of hearing thresholds. Thus, it provides an objective method for characterizing how much the TE changes relative to linear amplification because linear amplification does not modify the TE of a signal. This method provides a means to extend measures of HA processing beyond audibility.
Our goals were twofold: (1) Measure TE modification in clinically-fit HAs fit for infants and young children; and (2) Examine how TE modification is related to infant speech discrimination. Our working hypothesis was that a measure of TE modification at user settings will contribute to understanding the variance in infant speech discrimination.
Methods
Participants
Forty-seven children aged 9 to 27 months (M=11.3 months, SD=4.6 months; 22 males) with sensorineural HL and bilaterally aided. All participants used their aids during behavioral testing (programmed to DSL 5.0)11 per managing providers and verified with test box measures (Table 1).
Table 1. Demographics
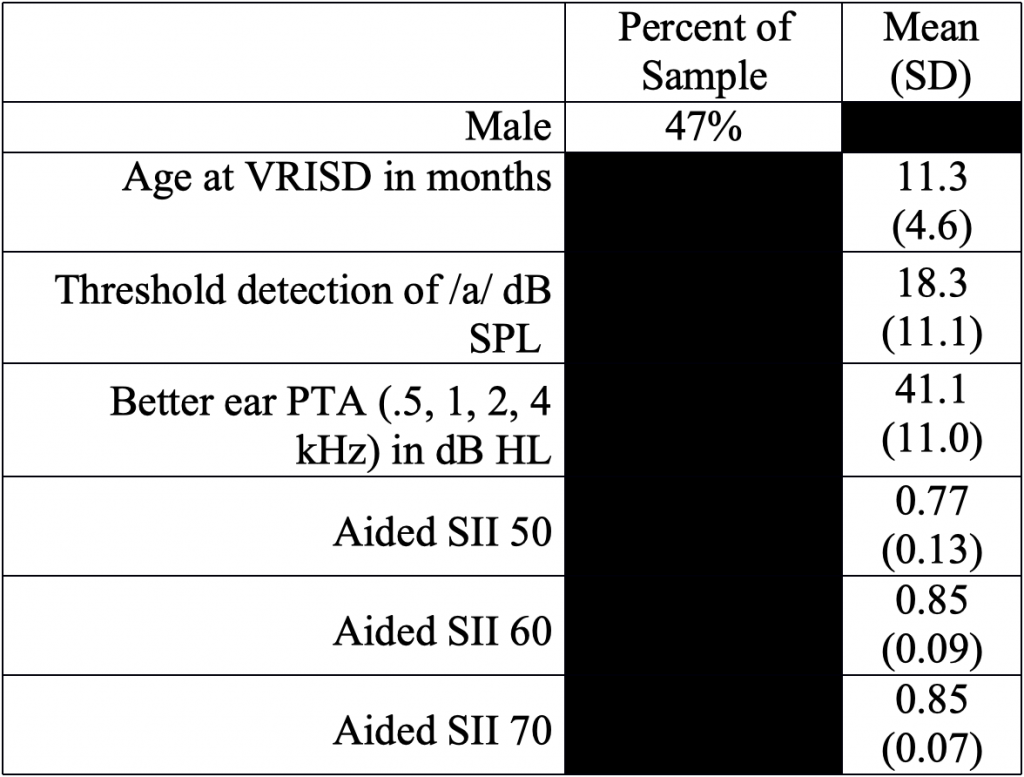
NOTE: Subject audiological characteristics and demographics. VRISD = visual reinforcement of infant speech discrimination; SII = speech intelligibility index.
Personal HAs and Acoustic Recordings
HAs from three manufacturers (18 models) were programmed with fitting data from the child’s better hearing ear to create the acoustic recordings. Full details regarding recording setup are available, see.12-14
Stimuli used for the HA recordings and acoustic analysis were two HINT15 sentences in quiet (one male and one female talker) at three presentation levels (PL): 55, 65, and 75 dB SPL.
TE Modification Calculation
CepCorr compares the time-frequency envelope modulation for HA output with that of a reference signal. Metric values range from 0 (no match in time-frequency envelope modulation between reference and HA-processed signal) to 1 (perfect match between signals). Full details on the calculation of CepCorr are available in Kates and Arehart.10
Behavioral Testing Protocol
Two contrasts (/a-i/ and /ba-da) were used in the behavioral assessment. These contrasts represent different levels of discrimination difficulty, with the vowel contrast (/a-i/) being easiest and the consonant contrast (/ba-da/) being most difficult.16-18
Testing is described in Uhler et al.,19, and is reviewed here. We employed visual reinforcement infant speech discrimination (VRISD), a conditioned head-turn oddball paradigm similar to that used for VRA. VRISD assesses an infants’ detection of a change in speech stimuli. The performance was reported in p(C)max, an unbiased value, derived from d-prime,20 taking into account correct responses and rejections, false positives, and misses. Criteria were met (i.e., discrimination was deemed successful) when an infant achieved a p(C)max ≥0.73. If the child achieved criterion for the contrast at 50 dBA, then testing for the first contrast was complete and testing for the second contrast was initiated.18,21 However, if the child did not reach criterion at 50 dBA, the level was increased to 70 dBA, and then the level was reduced to 60 dBA, regardless of performance at 70 dBA.
Results
TE Modification
A linear mixed-effects (LME) model was used to determine the effects of PL and four frequency pure-tone average (PTA; 0.5, 1, 2, 4 kHz) on TE modification (CepCorr) in quiet. A random intercept for ‘Fitting’ was included to account for the correlation of observations from the same infant’s record. An increase in PL from 55 dB to 75 dB resulted in an increase in TE modification by 0.065 (95% CI -0.05, -0.08; p=<0.001), whereas an increase in PL from 65 dB to 75 dB did not result in a significant change in TE modification (β=-0.008; 95% CI 0.02, -0.003 p=0.156). Also, a unit increase in PTA resulted in a significant increase in TE modification by 0.003 (95% CI -0.002 -0.004; p=<0.001). The significant effects of PL and PTA on TE modification are in agreement with previous studies and likely capture the effects of HA processing (e.g., WDRC) at higher PLs and greater degrees of HL.12,13
Speech Discrimination and TE Modification
We sought to identify how a measure of TE modification relates to speech discrimination performance in infants while utilizing their personal HAs. The VRISD score used for the analysis was the p(C) max score for the lowest intensity level where the infant reached criteria. For infants who did not reach criteria, we used their best p(C) max score regardless of PL. LME regression models with a random intercept for each participant were fit to accommodate the repeated measurements within an infant to examine the relationship of p(C)max with PL and TE modification. The /ba-da/ contrast had a VRISD score at 50 dBA that was approximately 0.08 lower than /a-i/ (95% CI -0.02, -0.07; p=<0.001), as measured by p(C)max. This finding indicates that overall performance on /ba-da/ was poorer than for /a-i/. Sixty percent of infants met criteria for /ba-da/ discrimination at either 50, 60, or 70 dBA, while 83% of infants met criteria for /a-i/ discrimination. The addition of TE modification in quiet at 55 dBA was not significant (β=-0.16; 95% CI -0.65, 0.32; p=0.516) and the estimate of the /ba-da/ contrast was unchanged, indicating that TE modification was not significantly related to performance, not explaining any of the variability in speech discrimination. These results were similar for all combinations of TE modifications and VRISD performance measured. Results are visualized in Figure 1, where there are tight bands for TE modification that span the entire range of behavioral performance, illustrating the limited ability to predict performance from TE modification.
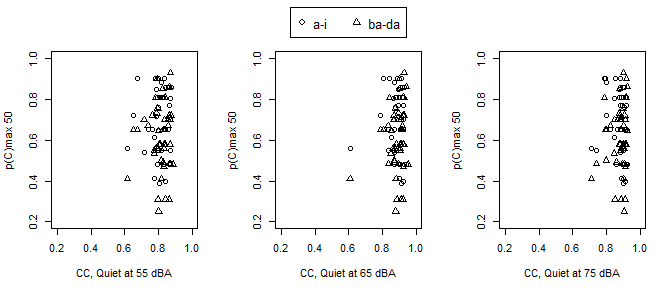
Figure 1. Behavioral performance (p(C) max 50) for the a-i and ba-da stimuli (see legend) as a function of TE. Each panel represents PL (in level from left to right).
Discussion
The use of TE modification does not explain variability for infants on this behavioral speech discrimination task. While increasing PL increased TE modification, this measure cannot be used to accurately predict behavioral performance for a task in quiet for infants. Overall infants continue to have better speech discrimination abilities for /a-i/ than /ba-da/, which has been reported previously in infants with HL,17,22 and our results are similar for /ba-da/ performance in NH infants.23 While infant speech discrimination is poorer for consonant contrasts than vowel contrasts, and test-retest reliability is a challenge,24 our results did not find a relationship between TE modification and discrimination as measured by the vowel versus the consonant contrast. We know that NH infants require additional information such as intensity,23,25 SNR26, and TE and TFS cues,6 for speech discrimination. Our findings suggest that TE modification in infants with HL does not account for differences in speech discrimination, as seen in adults with HL and NH.8,9
In this study, and previous work, we have shown that reliable TE modification measures can be captured from commercial HAs.14 These measures in the present study indicate that for children fit using best practices, including assuring audibility, there is little variation in the range of TE modification across individual HA fittings (see Figure 1). On one hand, this is ideal, as it indicates that clinical providers are following published guidelines. However, given the limited variability in TE modification, it is of limited use for predicting the wide variability in speech discrimination performance in this study population. These results paired with previous findings support exploring how infants with HL utilize both TE and TFS for speech discrimination.
Clinical Gems
Speech discrimination can be assessed in young children with HL.
TE modification does not predict behavioral infant speech discrimination.
The role of TE in speech discrimination is likely different for infants with HL than adults with HL.
Acknowledgments
Funding for this research was provided by the National Institutes of Health – National Institute on Deafness and other Communication Disorders 1K23DC01358; and by CCTSI=NIH/NCRR Colorado CTSI Grant Number UL1 TR001082 to author KU. We are grateful to the parents, participants, and audiologists who participated in this work.
References
- DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav Dev 1986. doi:10.1016/0163-6383(86)90025-1
- Mehler J, Jusczyk P, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition 1988. doi:10.1016/0010-0277(88)90035-2
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron 2010. doi:10.1016/j.neuron.2010.08.038
- Werker JF, Gervain J. Speech perception in infancy. The Oxford Handbook of Developmental Psychology, Vol. 1: Body and Mind. Philip David Zelazo. Ed. 2012:909–25.
- Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol 2015;66(1):173–96. doi:10.1146/annurev-psych-010814-015104
- Cabrera L, Werner L. Infants’ and adults’ use of temporal cues in consonant discrimination. Ear Hear 2017;38(4):497–506. doi:10.1097/AUD.0000000000000422.
- Warner-Czyz AD, Houston DM, Hynan LS. Vowel discrimination by hearing infants as a function of number. J Accoust Soc Am 2014;135(May):3017–24. doi:10.1121/1.4870700
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BCJ. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc Natl Acad Sci U S A 2006;103(49):18866–69. doi:10.1073/pnas.0607364103
- Hopkins K, Moore BCJ. The contribution of temporal fine structure to the intelligibility of speech in steady and modulated noise. J Acoust Soc Am 2009;125(1):442–46. doi:10.1121/1.3037233
- Kates JM, Arehart KH. The hearing-aid speech perception index (HASPI). Speech Commun 2014;65:75–93. doi:10.1016/j.specom.2014.06.002
- Scollie S, Seewald R, Cornelisse L, et al. The desired sensation level multistage input/output algorithm. Trends Amplif 2005;9(4):159–97. doi:10.1177/108471380500900403
- Kates JM, Arehart KH, Anderson MC, Muralimanohar RK, Harvey LO. Using objective metrics to measure hearing aid performance. Ear Hear 2018;39(6):1165–75. doi:10.1097/AUD.0000000000000574
- Souza P, Arehart K, Schoof T, Anderson M, Strori D, Balmert L. Understanding variability in individual response to hearing aid signal processing in wearable hearing aids. Ear Hear 2019;40(6):1280–92. doi:10.1097/AUD.0000000000000717
- Rallapalli V, Anderson M, Kates J, et al. Quantifying the range of signal modification in clinically fit hearing aids. Ear Hear 2019:1. doi:10.1097/aud.0000000000000767
- Nilsson M, Soli SD, Sullivan JA. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am 1994;95(2):1085–99. http://www.ncbi.nlm.nih.gov/pubmed/8132902.
- Boothroyd A. Auditory Perception of speech contrasts by subjects with sensorineural hearing loss. J Speech Hear Res 1984;27:134–44.
- Martinez A, Boothroyd A, Visser-Dumont L, Eisenberg L. Assessing speech pattern contrast perception in infants: Early results on VRASPAC. Otol Neurotol 2008;29:183–88.
- Uhler KM, Baca R, Dudas E, Fredrickson T. Refining stimulus parameters in assessing infant speech perception using visual reinforcement infant speech discrimination: sensation level. J Am Acad Audiol 2015;26(10):807–814. doi:10.3766/jaaa.14093
- Uhler KM, Hunter SK, Tierney E, Gilley PM. The relationship between mismatch response and the acoustic change complex in normal hearing infants. Clin Neurophysiol 2018;129(6):1148–60. doi:10.1016/j.clinph.2018.02.132
- Macmillan N, Creelman C. Detection Theory: A User’s Guide. (Lawrence Erlbaum Associates I, ed.). Mahwah, New Jersey; 2005.
- Nozza RJ. Thresholds are not enough: Understanding how infants’ Process. Speech has a role in how we manage hearing loss . In: Seewald RC, ed. A Sound Foundation through Early Amplification. Chicago, IL: Phonak AG; 2000:47–54.
- Uhler KM, Baca R, Dudas E, Fredrickson T. Refining stimulus parameters in assessing infant speech perception using visual reinforcement infant speech discrimination: Sensation level. J Am Acad Audiol 2015;26(10). doi:10.3766/jaaa.14093
- Nozza RJ. Infant speech-sound discrimination testing: effects of stimulus intensity and procedural model on measures of performance. J Acoust Soc Am 1987;81(6):1928–39. http://www.ncbi.nlm.nih.gov/pubmed/3611513.
- Cristia A, Seidl A, Houston D. Test – retest reliability in infant speech perception tasks. 2016;21(5):648–67. doi:10.1111/infa.12127
- Leibold LJ, Werner LA. Relationship between intensity and reaction time in normal-hearing infants and adults. Ear Heairng 2002;23(2):92–97. http://www.ncbi.nlm.nih.gov/pubmed/11951853.
- Nozza RJ, Wilson WR. Masked and unmasked pure-tone thresholds of infants and adults: development of auditory frequency selectivity and sensitivity. J Speech Hear Res 1984;27(4):613–22. http://www.ncbi.nlm.nih.gov/pubmed/6521469

