Do Hearing Aids Meet ANSI Standards?
Introduction
Quality control specifications for hearing aids have a long history dating back to the 1930s. You probably remember learning about ANSI S3.22 specifications (referred to herein as the “ANSI standard”) during your graduate school days, which are still used by the FDA to govern hearing aid quality control today. The ANSI standard is the only way that we as audiologists can ensure hearing aid quality control. Standardized electroacoustic testing is required of the manufactures but not of the dispensing professional despite being recommended best-practice by the American Speech-Language-Hearing Association and the American Academy of Audiology. Some professionals, however, may wonder if these procedures are unnecessary or if they are still applicable to today’s technology. The purpose of the ANSI standard is to make sure the main components of the hearing aid are functional by assessing the following: maximum power output, which describes the highest level of sound the hearing aid can produce, gain, which describes the highest level of amplification the hearing aid can produce, distortion, which gives us information about component integrity, equivalent input noise, which measures the extraneous noise produced by the hearing aid, and attack and release times, which measure how quickly gain changes are made as a function of input. ANSI also provides a tolerance for each of these measures, which is a range of values within which the hearing aid is still compliant with the ANSI standard. These ranges allow for slight variations that indicate the hearing aid is still operating as intended.
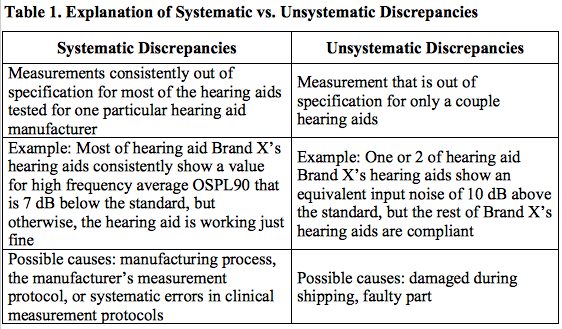
If you complete quality control measures on every hearing aid prior to dispensing, you may have noticed 2 different patterns of noncompliance with the standard: systematic and unsystematic discrepancies (Table 1).
Several previous studies have indicated that no more than 68% of hearing aids tested were in compliance with the standard.1–4 Rates in newer, digital hearing aids have not been reported though possibly due decline in the use of the standard for quality control. It is possible (likely) that the manufacturing process of hearing aids is now more reliable and that fewer faulty hearing aids are coming off the manufacturing line, which would result in a decrease in unsystematic discrepancies. However, the percentage of systematic discrepancies could have increased in modern hearing aids due to the ambiguity surrounding how to set advanced features (i.e. noise reduction) during quality control testing.
The ANSI standard is valuable for quality control, but doesn’t address other modern features such as directional microphones. There have been several reports of faulty directional microphone installation, and directional microphones are subject to debris and moisture, which can cause the microphone to fail over time.5 Therefore, it has been suggested that directional processing be tested for quality control purposes before the initial fitting and at subsequent follow-up visits.
Objectives
- Determine the percentage of new hearing aids in compliance with the relevant ANSI standard
- Determine the percentage of hearing aids with functional directional processing
- Assess trends associated with compliance across hearing aids, specifications, and manufacturers
PROCEDURE
Hearing Aids
Seventy-three (73) new behind-the-ear (BTE) hearing aids were tested consecutively after they were received in the Vanderbilt Bill Wilkerson Audiology Clinic using Audioscan’s Verifit hearing instrument verification system (Software version 3.1)6 and Frye’s Fonix 8000 test box system.7 The number of hearing aids tested per brand was as follows: 22 for Brand 1; 22 for Brand 2; 13 for Brand 3; and 16 for Brand 4. Brands 1, 2, and 4 were all standard BTE style hearing aids. Brand 3 was a receiver in the canal style hearing aid.
Testing Considerations
Several specific protocol considerations were required to properly test the hearing aids based on individual manufacturer differences such as availability of a “test” setting. Generally, these specific protocols were difficult to locate, not well publicized by the manufacturer, and in some cases required contact with multiple individuals within a company.
ANSI Hearing Aid Test
Each hearing aid was run through the ANSI AGC test protocol.8 The ANSI standard and the manuals for the verification systems provide specific instruction for how to complete this test. The measured values for each component were compared to the manufacturer’s specification for the device. Each device was assigned a “pass” or “fail” for each ANSI component, based on the specified tolerance.
Directional Processing Testing
Testing of directional processing was performed to assess functionality using the Audioscan Verifit test system. Directional processing in the test box was measured according to instructions in the Verifit manual.6 The difference between the coupler output for signals presented from the front and back speakers (front-to-back separation) was calculated and recorded.
RESULTS
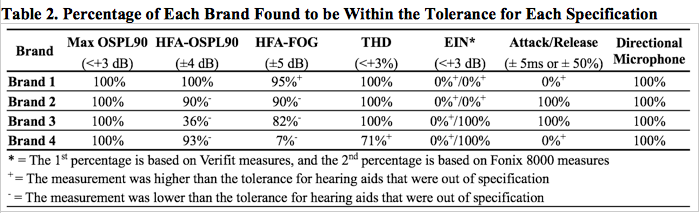
Table 2 displays the percentage of hearing aids within specification for each measurement as a function of brand. Out of the 73 hearing aids tested, none were within the allowable tolerance for every specification. In fact, only the Max OSPL90 measure had no instances of noncompliance. In general, the trends of noncompliance were systematic across each brand, as unsystematic discrepancies were only responsible for approximately 7% of the total instances of noncompliance.
The EIN, or the circuit noise of the hearing aid, was measured for each manufacturer and the corresponding tolerance limits are shown in Figure 1. Out of the 73 hearing aids tested, not a single hearing aid was within specification for EIN. EIN measurements were cross-checked using the Fonix 8000. EIN measurements in the Fonix 8000 were very similar to the Verifit measures for Brand 1 and Brand 2. Brand 3 and Brand 4, however, showed significant differences between the 2 testing systems. All 4 brands were noncompliant when tested using the Verifit; however, when using the Fonix 8000, Brand 3 and Brand 4’s EIN measurements were compliant with the standard suggesting that Brand 3 and Brand 4 were possibly overestimated due to the noise floor of the Verifit system.
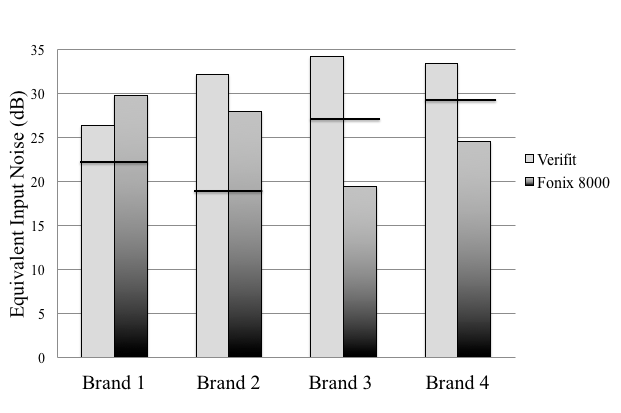
Figure 1. Equivalent input noise measurements obtained using the Verifit and Fonix 8000 are shown for each manufacturer. The black line indicates the limit of the tolerance. Values below this line are compliant, and values above this line are noncompliant.
All of the hearing aids’ directional processing was found to be functional as exemplified by an average separation between the responses for front and back signal locations of at least 4 dB for each instrument shown in Figure 2. Although it appears that there is a greater front-to-back separation for Brand 2, this should not be interpreted as providing more benefit for the patient, as the test box does not simulate a real-life situation; this test is only a measure of quality control.
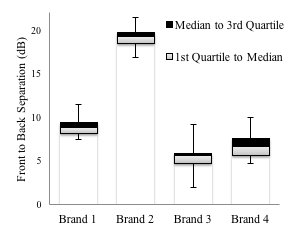
Figure 2. The results of the directional microphone testing are shown. The front-to-back separation in decibels is shown for each brand. The whiskers indicate the range of the data.
DISCUSSION
Unsystematic Noncompliance Issues
This study revealed that the 4 hearing aid brands tested were largely noncompliant with all aspects of the established ANSI standard. In an effort to determine the reason(s) for the noncompliance, 2 hearing aids of a differing model were analyzed from each brand. The results of this additional analysis revealed that the noncompliance was largely consistent across both models within each brand. This finding suggested that the noncompliance was largely a result of systematic discrepancies specific not only to a model, but also to a manufacturer. Only a small percentage (2–11% depending on brand) of hearing aids were found to have unsystematic compliance issues associated with more traditional quality control issues (Figure 3). If we assume that the data from the previously mentioned studies from the 1980s1–4 represent largely unsystematic compliance issues, quality control has become significantly better. However, there remains a significant percentage of modern, digital hearing aids with quality control issues.
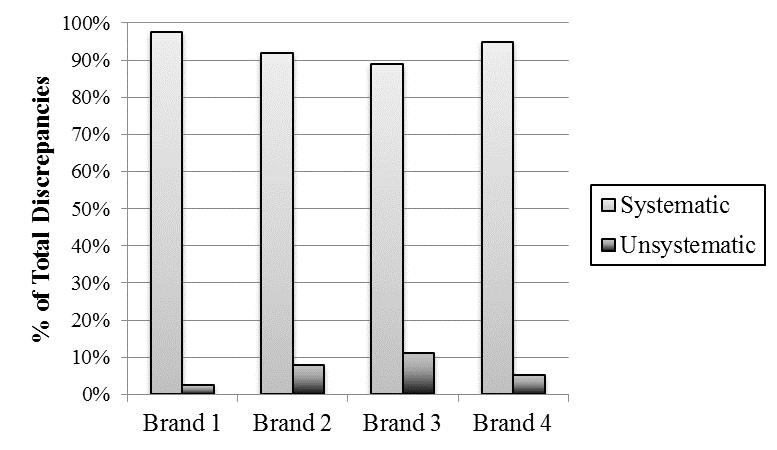
Figure 3. The proportion of systematic and unsystematic discrepancies between our measurements and the manufacturers’ specification are shown for each brand.
Systematic Noncompliance Issues
The majority of noncompliance issues were systematic in nature, suggesting particular brands are prone to specific quality control issues or systematic measurement errors. The source of these issues could be in the manufacturing process, the manufacturer’s measurement protocol, or systematic errors in clinical measurement protocols (see Table 1).
To investigate potential sources of systematic noncompliance issues that originated from the manufacturer, each hearing aid manufacturer was contacted to investigate potential reasons for noncompliance. When a representative from Brand 2 was contacted to inquire about their EIN measurement being significantly out of specification using the Verifit and the Fonix 8000, it was suggested that our measurements were higher because their published specifications are based on measurements taken inside of an anechoic chamber. The requirement of testing in an anechoic chamber would preclude measurement of quality control in virtually all clinical settings. Specifically, if the purpose is quality control, it is crucial that a testing method is viable in a typical clinical setting.
Directional Processing
All of the hearing aids tested in this study were found to have directional processing that functioned as expected. However, we still encourage clinicians to verify directional processing prior to fitting the hearing aid for quality control purposes and to provide an important benchmark against which the function can be compared at subsequent patient visits since directional processing has the potential to shift over time.
The important quality control aspect of this measure is how consistent the front-to-back separation value is across instruments within a manufacturer.9 Our results indicate that quality control for directional processing results in largely consistent directional effects; however, Brand 3 appeared to have more variation than the other 3 brands, suggesting quality control of the directional processing was slightly poorer for this model.
Equivalent Input Noise
Equivalent Input Noise (EIN) was the measurement found to be most frequently out of specification for the hearing aids assessed in this study. This routine failure in meeting published EIN standards is consistent with the findings of another recent study.10 As pointed out by those authors, the EIN measure often is not an accurate indication of the potential audibility of internal noise. However, clinicians still need to be able to better replicate the EIN test protocols clinically for quality control purposes.
Clinical Implications
The systematic noncompliance prevalent in this study supports the importance of clearly defined and established test protocols for ANSI testing by all manufacturers. Further, these protocols should be clinically viable so that accurate quality control testing can be completed. If the protocol is not published, a new or inexperienced hearing aid dispenser may presume that a noncompliant device is defective and return it to the company, when, in fact, there may have just been a difference in quality control testing protocol. This is problematic because returning the device requires professional time, is inefficient and inconvenient for patients, and can damage patient-clinician rapport. The safest procedure for practicing clinicians would be to return instruments to the manufacturer in cases of unsystematic noncompliance, particularly when instruments fall well outside the reported standard. However, to ensure that functioning instruments are not inadvertently returned to the manufacturer, it is equally incumbent on the manufacturer that standards reflect not only expected values, but also include the range of values beyond which mechanical dysfunction is expected. The purpose of having a quality control standard is to ensure that patients receive devices that are free from defects and consistent in the acoustic properties provided. If these properties are not accurately reflected in the current tolerances, then these tolerances should be adjusted to avoid the wasted resources incurred by returning instruments that are out of specifications, but are free from defects and provide patients with a consistent listening experience.
CONCLUSIONS
- Quality control measures of hearing aids are still a critical component to providing best-practice patient care.
- To complete quality control procedures accurately, manufacturers are encouraged to provide clinically replicable, well-defined protocols.
- Clinicians are encouraged to routinely perform ANSI testing on new hearing aids to check for systematic and unsystematic discrepancies and to optimize patient care.
REFERENCES
- Bess F. Condition of hearing aids worn by children in a public school setting. In: The Condition of Hearing Aids Worn by Children in a Public School Program. Washington: US Department of Health Education and Welfare; 1977.
- Chial M. Electroacoustic assessment of children’s hearing aids: Repeatability of measurement and determination of merit. In: The Condition of Hearing Aids Worn by Children in a Public School Program. Washington: US Department of Health Education and Welfare; 1977.
- Townsend TH, Olsen CC. Performance of new hearing aids using the ANSI S3.22-1976 standard. J Speech Hear Disord 1982;47(4):376–82. http://www.ncbi.nlm.nih.gov/pubmed/7186579.
- Callaway SL, Punch JL. An electroacoustic analysis of over-the-counter hearing aids. Am J Audiol 2008;17(1):14–24. doi:10.1044/1059-0889(2008/003).
- Frye G. Understanding the ANSI standard as a tool for assessing hearing instrument functionality. Hear Rev 2005;12(5):22.
- Audioscan Verifit. Verifit User’s Guide Version 3.10. 2012.
- Frye Electronics Inc. Fonix 8000 Manufacturers Specification and Information. 2007.
- American National Standards Institute. Specification of hearing aid characteristics (ANSI 3.22-2009). 2009.
- Dillon H. Special Hearing Aid Issues for Children. 2nd ed. New York: Thieme Medical Publisher; 2012.
- Lewis JD, Goodman SS, Bentler RA. Measurement of hearing aid internal noise. J Acoust Soc Am. 2010;127(4):2521–28. doi:10.1121/1.3327808.

