Superior Semicircular Canal Dehiscence
Superior canal dehiscence (SCD) is a fascinating disorder of relevance to every audiologist and otolaryngologist. The condition is characterized by the abnormal connection of the superior semicircular canal (SCC) to the intracranial space. The superior semicircular canal hugs the dura, and is the only one of the three semicircular canals not to be surrounded by air. Figure 1 shows a reconstruction from a CT scan, of the anatomy and relationships of the SCC. Normally, the bony labyrinth totally encloses the fluids of the inner ear, except for the openings into the capsule by the round window (RW), oval window (OW), and smaller openings for the vestibular aqueduct and cochlear aqueduct (Figure 2).
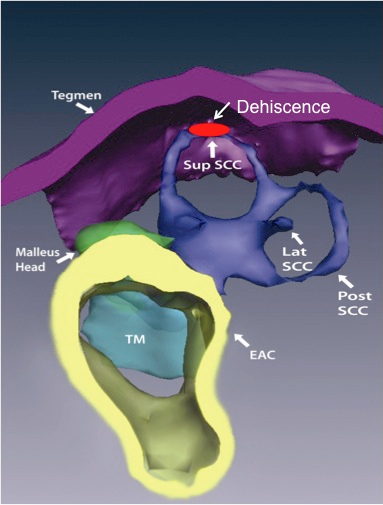
Figure 1. A reconstruction of the anatomy of the ear, showing the location of the dehiscence in the bony capsule of the labyrinth.
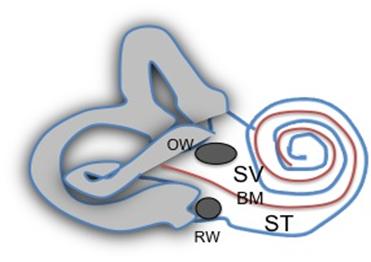
Figure 2. The bony labyrinth is pierced by two main openings, the oval window (OW) and the round window (RW), the red basilar membrane (BM) divides the ear into a scala vestibuli (SV) and scala tympani (ST) portion. The vestibular portion is grey, and the cochlear portion unfilled.
The OW opens on one side of the basilar membrane, on the scala vestibuli (SV) side, and this side also connects with the vestibular structures. The RW opens in the scala tympani (ST) side of the basilar membrane, and is the “relief” valve for the OW, as the inner ear fluids are incompressible. Normally, the RW total volume release is the same as the OW total volume displacement, as compared to the low compliance of these two structures, there are no other significant compliant areas in the normal bony labyrinth.
SCD introduces a new opening in the loop of the superior semicircular canal, in the portion of the inner ear with the vestibular structures, and connected to the scala vestibuli part of the cochlea. This changes the fluid mechanics in the inner ear in both the vestibular and the cochlear partitions. This kind of extra opening is sometimes referred to as a “3rd window,” such as in addition to the two other windows the OW and RW. There are other possible 3rd windows, which we will discuss later.
SCD was identified by measuring the vectors of eye movements in response to sound stimulation of the ear in affected canal, and finding that it aligned with plane of the SCC. Ewalds’ first law states that the eye movements are in the plane of the stimulated canal, and so the direction of evoked eye movements can point to the eliciting canal.1,2
Demographics
The prevalence of SCD is difficult to accurately identify. In a radiologic study of coronal computed tomography (CT) scans, it was found in between 3%3 and 9%4 of temporal bones scanned. In cadaveric temporal bone studies, incidences of between 0.4%5 and 0.5%6 have been found. There does not seem to be a sex bias, and most patients present in middle age. SCD dehiscence is bilateral in 20–50% of cases.7
Clearly, with this kind of radiologic and pathologic incidence, we should be seeing a lot more clinical SCD than is the actual experience, so it is very likely that many of these SCD do not cause symptoms, or if they do, they are so mild as to not cause the patient to seek medical attention.
Etiology
The current main proposed mechanism for SCD is that it is due to an underlying developmental abnormality, with poor and incomplete formation of the tegmen.8 The radiologic incidence is higher in children,9 which may point to a failure of maturation and thickening of the tegmen with aging in those patients who exhibit this finding in adulthood. With time, this abnormally thin bone over the SCC may be eroded by brain pulsations, a forced valsalva manoeuvre, or by minor head trauma. This later inciting event can sometimes be a point of litigation. Even in the presence of a bony opening into the canal radiologically, it is not uncommon to see a lack of symptoms, so a bony dehiscence may still be plugged by dura or other soft tissue, and not result in a physiological 3rd window.
Pathophysiology
Normally, bulk fluid flow with sound stimulation occurs between the OW and the RW. The fluid flow and associated pressure wave move the basilar membrane. Any other window could result in some dissipation of the pressure into that 3rd window, and some bulk fluid flow into the abnormal window. There are a number of possible “3rd” windows in the normal inner ear, including the vestibular aqueduct, the cochlear aqueduct, and the foramina of the blood vessels. It is thought that in the normal cochlea, the impedance of these windows is so high compared to the RW and OW that they are functionally closed,10 but this may not be the case in pathologic inner ears such as in Enlarged Vestibular Aqueduct Syndrome (EVAS). Indeed, the term “conductive hearing loss” is itself a bit of a misnomer, and implies an etiology based on inadequate conduction to the inner ear. I have advocated for differences in air and bone conduction thresholds to be better be described as “mismatch of air-bone thresholds (MABT).”11
A third window on the vestibular side of the basilar membrane (i.e. the scala vestibuli, the vestibule, or any of the semicircular canals) will dissipate energy from the scala vestibuli side of the BM, such as the side first compressed when the OW moves inward with sound or fistula testing; the dissipated pressure then cannot contribute to generating a pressure gradient across the BM (Figure 3).
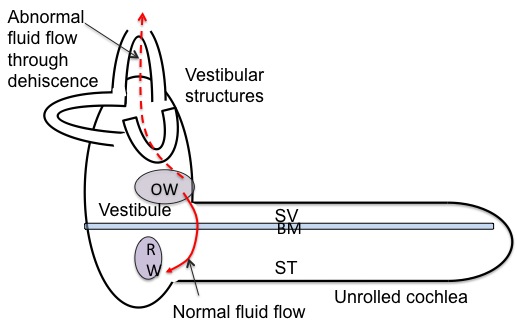
Figure 3. Normal fluid flow from OW to RW, and abnormal fluid flow from OW to the SCD. BM = basilar membrane; OW = oval window; RW = round window; ST = scala tympani; SV = scala vestibule.
This misdirected fluid and pressure dissipation will take away from BM displacement, and so will elevate air conduction thresholds, and is part of the reason a conductive hearing loss arises from these kinds of 3rd window lesions. A second mechanism contributing to a conductive hearing loss is enhancement of the bone conduction thresholds. This may arise from two mechanisms. The first is that at low frequencies, most of the movement of the BM in bone conduction is due to the inertia of the inner ear fluids, as the skull vibrates, the inner ear fluids want to stay in place. They can do this partly because the OW and RW can move inwards and outward and allow them to do so, but the impedance of the OW is the limiting factor as it is much higher than that of the RW. If the SV side of the basilar membrane has a low compliance 3rd window in it, then the fluid is allowed to “stay in place” much more effectively, and there is greater relative movement of the fluid and the surrounding bone, and greater movement of the BM (which is attached to the surrounding bone) with skull vibration. Another mechanism of bone conduction enhancement has been proposed, based on the compression of the cochlea by bone conduction,11 but compressional mechanisms of bone conduction don’t really become important till higher frequencies,12 whereas the borne conduction enhancement occurs mostly at low frequencies.
In a model such as this, a 3rd window on the scala tympani side should not have any effect on the hearing thresholds, as there is no change in the trans-BM pressure differential from fluid dissipation through here, as it is part of the normal pressure and fluid flow pathway anyway.
It is not clear why some patients present primarily with auditory symptoms, some with vestibular symptoms, and some with both. One study13 suggests that larger dehiscences present with both auditory and vestibular symptoms, whereas smaller ones presented with one or the other.
Vestibular stimulation may also occur, because the acoustic energy takes an aberrant path, so that more is directed towards vestibular structures than normally would be. For instance in SCD, the OW inward displacement, such as from positive pressure in the EAC or sound, results in fluid flow through the bony ampulla of the superior canal, past the endolymphatic ampulla, which would result in stimulation of the SCC, since cupular deflection away from the utricle (ampullofugal) is stimulatory in the vertical canals. This stimulates the superior semicircular canal, and so would cause an in-torsion and upward slow phase eye movement from Ewald’s first law which states that the direction of the elicited eye movement is the plane of the stimulated canal. The resetting eye movement when pressure is released, is then downbeat and ex-torsion. Sometimes a purely vertical movement only is seen. For a negative pressure in the EAC or for a valsalva which pushes on the dehiscence, the opposite movement would be seen. Figure 4 shows the typical eye movement on pressurization of the external ear canal.
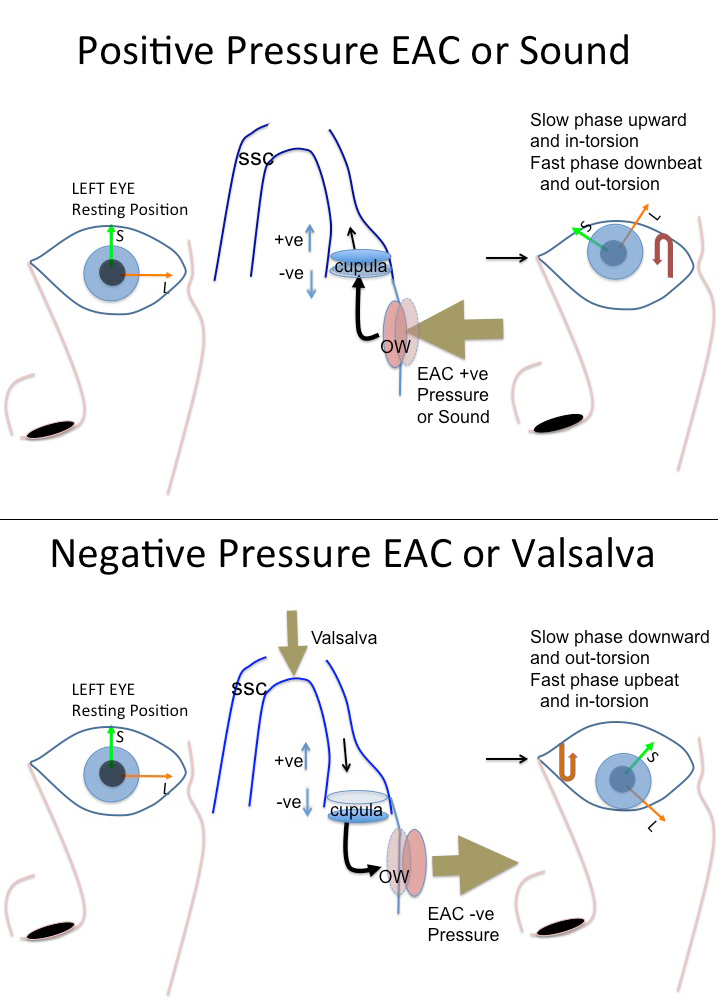
Figure 4A (top) and 4B (bottom). Typical eye movement with pressurization of the external ear canal. This represents the left eye, and positive pressurization of the left ear (Figure A) results in an upward and nasally directed (in-torsion) torsional eye movement. The resetting on release of pressure will be the opposite, i.e. out-torsion and a downbeat eye movement, which may be easier to see. Negative pressure or valsalva (Figure 4B) results in the opposite movement. SCC = superior semicircular canal; +ve represents an increase in firing rate as cupula moves ampullogual; and –ve a decrease in firing rate as cupula moves ampullopetal. OW = oval window, EAC= external ear canal.
From Ewalds second law, positive pressure in the EAC elicits a larger response (cupular nerve excitation) than negative pressure in the EAC (cupular nerve inhibition).
Symptoms
Patients with SCD can present with a variety of confusing symptoms. Often, they are as perplexed about their symptoms, and some symptoms such as hearing their eyes moving seem so bizarre to them, indeed they may think they are suffering from an obscure psychiatric disorder. It can be a great relief to them when they are finally given a diagnosis.
Typical symptoms and signs are those related to their autophony, and to the vestibular symptoms (Table 1).
Table 1. Typical History
1. Autophony
- Own voice echoing in ear
- Hear eyes moving or blinking
- Hear footsteps loudly in ear
- Hear body sounds such as neck vertebrae clicking
- Pulsatile tinnitus
2. Vestibular symptoms
- Dizziness on straining
- Dizziness with loud sounds
- Dizziness if pressurizes own ear
Typical Findings
- Weber materializes to affected ear
- Tuning fork held on ankle is heard in affected ear (128 Hz)
- Positive pressure in ear canal causes upward and intorsion of eye
- Straining causes opposite eye movement
The heightened awareness of body sounds is attributed to the hyper-sensitivity to bone conduction, although the pulsatile tinnitus is likely from the direct access brain pulsations have to the inner ear through the SCD. Hearing the voice echoing in the ear is a common symptom (autophony), and this gets better on lying down, similar to patulous eustachian tube. This may possibly occur because the brain swelling from lying down as venous return increases may plug the SCD. Patulous eustachian and SCD can be hard to tease apart. Patients may hear their eyes moving in a quiet room, but more often in my experience, can hear themselves blinking. They may also complain that when they walk on hard surfaces, or jog, their footsteps pound in their ears. Some patients can also hear normal body sounds such as neck vertebrae clicks and pops, or even bowel sounds.
The other major group of symptoms arises from abnormal stimulation of the vestibular system. It is not uncommon for patients to complain of non-specific lightheadedness, fogginess, or dizziness. More specifically, they may complain of Tulio’s phenomenon and loud sounds such as ambulance siren or a child crying may make them very unsteady. Straining, either in the bathroom or when lifting something heavy, or on coughing may also make them dizzy. A few patients have a habit of putting their fingers in their ear to clean their ear, and may “autofistula test,” and find that this pressurization makes them dizzy. In severe cases, they the visual environment may move in time with their heartbeat, as brain pulsations stimulate the inner ear, and cause eye movements (pulsatile oscillopscia).
Signs
Signs found on examination can help confirm the diagnosis. Tuning fork tests may strongly lateralize to the affected ear, unless there is bilateral disease. A tuning fork held on the ankle may travel up the body, and be heard in the affected ear. This is generally best tested with a 128 Hz tuning fork, in my own experience.
The pathognomonic test is looking for eye movements induced by fistula testing, i.e. on pressurization of the external ear canal. In this case (see Figure 4), due to stimulation of the superior canal, there is an in-torsional (away from the pressurized ear) and upward eye movement.
Straining may show the reverse eye movement, although this is less commonly seen easily. It may be difficult to see the eye movement, and VNG glasses are recommended. Alternatively the patient can be asked to look at a bright sharply defined object, such as an LED light, and asked if the light moves on pressurization of the external ear canal. Many patients who are symptomatic don’t exhibit this finding on visual inspection. It is possible that more sensitive tests such as scleral search coils or VNG may pick this eye movement up more often. Another useful way to enhance sensitivity is to look at the eye under the microscope (without shining the light directly in the eye!).
Various other disorders may also cause a positive fistula test, although the eye movements described above are specific to SCD. These include a lateral canal fistula from cholesteatoma, perilymphatic fistula (a controversial diagnosis except in clear trauma), and syphilis.
Investigations
Radiologic
CT scanning is the imaging modality of choice for SCD. Coronal cuts can be adequate, but reconstruction in the plane of the canal (Porshl’s plane) or at 90 degrees to the canal (Stenver’s plane) can help with the sensitivity (Figure 5). CT scans should be collimated to 0.5 mm if possible. Very thin bone cover (0.1mm or less) over the SCC may be confusing and difficult to interpret.14 Figure 5 shows a typical coronal CT scan, showing the dehiscence on both sides.
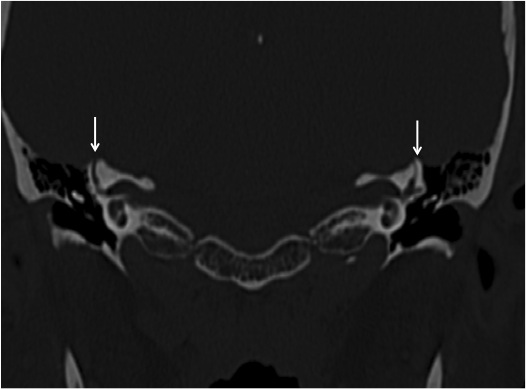
Figure 5. Coronal CT scan showing bilateral SCD, highlighted by arrows.
Audiogram
Typically, SCD causes a low frequency conductive loss. Most often, the conductive loss is mild, and in the low frequencies only. Figure 6 shows a typical audiogram in SCD. Whether this can ever be large enough to be confused with a true otosclerotic type hearing loss is controversial. In temporal bone experiments, even when the SCD was open to air, losses were only below 1 kHz, and when covered with dura, the losses were minimal.15
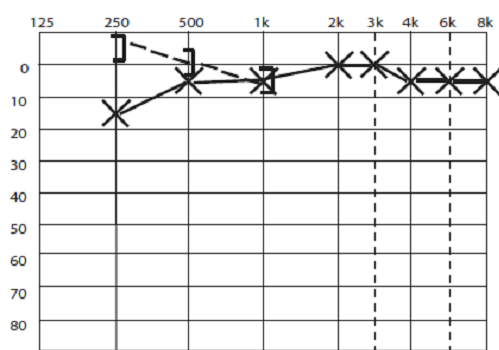
Figure 6, typical audiogram in SCD, showing low frequency conductive hearing loss, and supranormal bone conduction thresholds for bone conduction in the lowest frequencies.
Some red flags in the audiogram are listed in Table 2.
Table 2.“Red Flags” for a 3rd Window Lesion on an Audiogram
- Low frequency conductive hearing loss with otherwise normal hearing
- Bone conduction better than 0 dB HL in low frequencies
- Presence of stapedial reflex despite conductive hearing loss
- Dizziness on pressurization for tympanometry
- Dizziness on loud sound presentation for acoustic reflex testing
- Weber strongly materializing despite little asymmetry in hearing test
Other intriguing findings reported have been a small increase in the tympanometric compliance in affected ears compared to the contralateral ear, particularly in the presence of a large conductive hearing loss.16 This would be in keeping with the reduced input impedance of the ear as measured with umbo laser doppler vibrometry recordings.17
VEMPS
One of the most commonly used diagnostic tests for SCD are VEMPs. These have been shown to have a lowered threshold in SCD, both for air conduction and bone conduction.18 It is postulated that the fluid flow past the saccule towards the 3rd window stimulates this structure. VEMPS can be of two types, cervical, or cVEMPS (collected from a tonically contracted sternocleidomastoid muscle as it is an inhibitory response) or ocular, or oVEMPS, collected from the extra ocular muscles by an electrode below the eye. It is thought the oVEMPS are more dominated by the utricle and the cVEMPS more by the saccule, but both contribute to the responses,19 but this remains unclear, and the different otolith organs may also be differentially stimulated by different frequencies of stimulation.20
Both responses occur both ipsilaterally and contralaterally, with the cVEMPS ipsilaterally being characterized by p13n23 responses, and for the oVEMPS ipsilateral n13 responses. These early peaks are the only ones that are thought to be of vestibular origin.
Typical settings for VEMPS include either low frequency tone bursts or clicks at 5 Hz positive polarity, and tones of 500 Hz positive polarity with rise/fall times of 1ms and 2 msec plateau. The bandpass filtering is typically set to 20−2000 Hz, and traces are averaged about 100 times. Figure 7 shows typical VEMP responses from a subject with SCD in the left ear, with responses down to 65 dB sound presentation level.
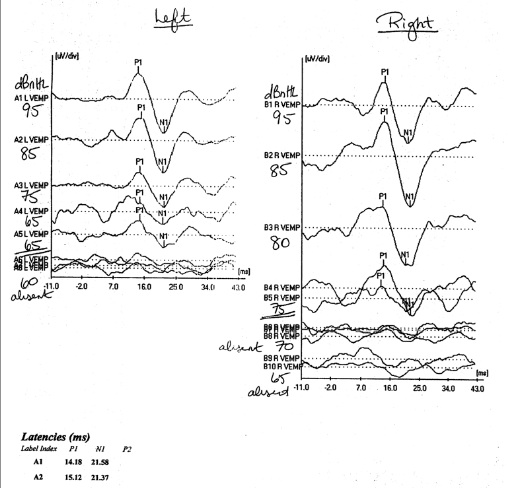
Figure 7. cVEMPs in a subject with bilateral SCD, right more symptomatic than left. The VEMP response can be seen to persist down to stimulation levels as low as 65 dB HL on the right side, whilst lost past 75 dB on the right.
Some studies have also reported increased SP/AP ratio in ECochG similar to Meniere’s Disease in SCD.21,22
Differential Diagnosis
Several disorders can cause similar symptoms to SCD. Of particular note, as mentioned earlier, another common cause of autophony is patulous eustachian tube, and this may mimic SCD very closely, in that it also gets better with lying down, and gives almost identical autophony symptoms, although in more severe cases patulous eustachian tube subjects will also hear their breathing. There are several other causes of conductive hearing loss with do not arise from the middle ear. These are listed in 3rd windows in the inner ear, some of which are listed in Table 3.
Table 3. Non-middle Ear Causes of Conductive Hearing Loss
- X-linked Stapes linked stapes gusher syndrome (DFN-3)
- Inner ear abnormalities such as EVAS
- Calibration error
- Collapsing canals.
- Non-organic hearing loss
-All of the above also will result in present stapedial reflexes in the presence of a conductive hearing loss. A middle ear condition that will also cause reflexes to be present in the presence of a conductive hearing loss is: - Stapedial crura fracture proximal to insertion of the stapedius tendon.
Other 3rd window etiologies
- Posterior canal dehiscence11,23,24
- Lateral canal dehiscence - usually by cholesteatoma, so difficult to determine cause of conductive hearing loss
- Enlarged vestibular aqueduct
- X-linked stapes gusher (DFN-3)
- Cochlea carotid fistula25
- Fallopian canal cochlear fistula26
- Pagets disease of bone10
- Inner ear abnormalities in which the scala vestibuli opens into the IAC
Treatment
Treatments can be divided into conservative and surgical. For many patients, an explanation is all that is required for them to continue comfortably with their symptoms. For some, who have pressure induced symptoms, such as driving over a hill or while riding in elevators, a tympanostomy tube may help alleviate these pressure symptoms by limiting TM excursions during pressure changes. For others, with predominately pulsatile tinnitus type or autophony symptoms, a diuretic may reduce intracranial pressure enough to alleviate their symptoms to a livable level.
Surgical treatments have mainly been focused on either resurfacing or plugging the SCC. There are a number of approaches to these methods, the SCC can be approached through a middle fossa craniotomy, and exposed from above by lifting up the temporal lobe. This gives good exposure of the canal, and this exposure can be used to resurface or plug the canal accurately. Usually plugging is performed for both the anterior and posterior limbs of the canal.27 The risks of this kind of approach include the small but real risks of intracranial violation, such as a CSF leak, infection and meningitis, and a small risk of seizure.
A second approach is through the mastoid itself, an approach familiar to most otologists. The SCC is exposed and outlined, and often there is a defect in the tegmen already present at the site of dehiscence. Through this approach, the SCC can also be plugged. Myself and others28,29 have described an approach to resurface the SCC though the mastoid as well. Figure 8 shows an intraoperative photograph from a SCD resurfacing performed transmastoid, the dehiscent canal can be seen in the mirror held above it, holding the dura up away from it. In general, while the resurfacing approaches are theoretically more physiologic in that they don’t defunction the canal, it’s possible that some resurfacing works by accidently plugging the canal. Plugging, theoretically has a greater risk of hearing loss as well, because it is a greater violation of the otic capsule. There is a real risk of hearing loss after SCD repair, which varies quite widely from series to series but is in the order of 10−15% for primary cases, and higher for revisions. In the middle fossa approaches, plugging has been found to be more stable over time than resurfacing.
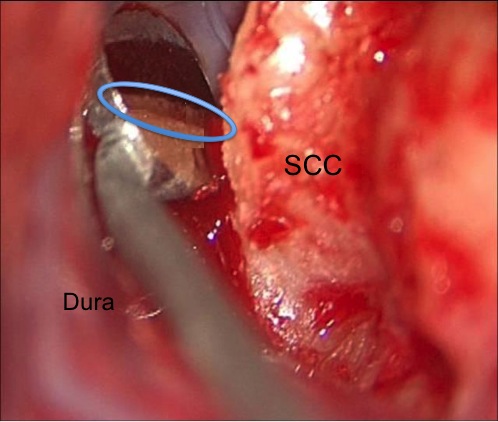
Figure 8. Intraoperative photo of dura being held away from SCC by a small mirror, and the dehiscence is seen in the mirror reflection (in outlined oval).
More recently, RW (and sometime OW) plugging have been described as treatments for SCD.30 This has the effect of stiffening the compliance of the inner ear. Theoretically this should increase the input impedance from of the inner ear as seen from the middle fossa, and so decrease pulsatile tinnitus, but it has been reported to also help autophony and Tulio’s.
Summary
SCD is an increasingly recognized condition, and one that every audiologist ought to be aware of. The symptoms can be subtle and easily missed in some patients. They can also overlap with other disorders such as hyperaccusis, patulous Eustachian tube or traumatic perilymphatic fistula. There are cues that the audiologist may detect in the audiogram that may be the first signs pointing towards this diagnosis. SCD is an important disorder to detect, as it is potentially repairable, and the patient’s quality of life dramatically improved. Referral to an interested otolaryngologist is recommended for symptomatic patients.
References
- Minor LB, Solomon D, Zinreich JS, et al. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg 1998;124:246–58.
- Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology 2000;55:1833–41.
- Masaki Y. The prevalence of superior canal dehiscence syndrome as assessed by temporal bone computed tomography imaging. Acta Otolaryngol. 2011 Mar;131(3):258–62. doi: 10.3109/00016489.2010.526145. Epub 2010 Dec 10. PubMed PMID: 21142747.
- Williamson RA, Vrabec JT, Coker NJ, Sandlin M. Coronal computed tomography prevalence of superior semicircular canal dehiscence. Otolaryngol Head Neck Surg 2003;129:481–9.
- Tsunoda A, Terasaki O. Dehiscence of the bony roof of the superior semicircular canal in the middle cranial fossa. J Laryngol Otol 2002;116(7):514–8.
- Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg 2000;126:137–47.
- Belden CJ, Weg N, Minor LB, Zinreich SJ. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology 2003;226:337–43.
- Chien WW, Carey JP, Minor LB. Canal dehiscence. Curr Opin Neurol 2011;24(1):25–31. doi: 10.1097/WCO.0b013e328341ef88. Review. PubMed PMID: 21124219.
- Chen EY, Paladin A, Phillips G, et al. Semicircular canal dehiscence in a pediatric population. Int J Pediatr Otorhinolaryngol 2009;73:321–327
- Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol 2008;29(3):282–9. doi: 10.1097/mao.0b013e318161ab24. Review. PubMed PMID: 18223508; PubMed Central PMCID: PMC2577191
- Bance M. When is a conductive hearing loss not a conductive hearing loss? Causes of a mismatch in air-bone threshold measurements or a "pseudoconductive" hearing loss. J Otolaryngol 2004 Apr;33(2):135–8. Review. PubMed PMID: 15518108.
- Stenfelt S, Goode RL. Bone-conducted sound: physiological and clinical aspects. Otol Neurotol. 2005;26(6):1245–61. Review. PubMed PMID: 16272952.
- Pfammatter A, Darrouzet V, Gartner M, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol 2010;31:447–54.
- Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol 2007;28:920–26.
- Luers JC, Pazen D, Meister H, et al. Acoustic effects of a superior semicircular canal dehiscence: a temporal bone study. Eur Arch Otorhinolaryngol 2014 Jan 1. [Epub ahead of print] PubMed PMID: 24381023.
- Castellucci A, Brandolini C, Piras G, Modugno GC. Tympanometric findings in superior semicircular canal dehiscence syndrome. Acta Otorhinolaryngol Ital 2013 Apr;33(2):112–20. PubMed PMID: 23853402; PubMed Central PMCID: PMC3665379.
- Rosowski JJ, Songer JE, Nakajima HH, et al. Clinical, experimental and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol 2004;25:323–32.
- Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 2008;70:464–72.
- Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. ClinNeurophysiol 2010;121:132–44.
- Dennis DL, Govender S, Chen P, et al. Differing response properties of cervical and ocular vestibular evoked myogenic potentials evoked by air-conducted stimulation. Clin Neurophysiol 2013; pii: S1388–2457(13)01169-3. doi: 10.1016/j.clinph.2013.11.001. [Epub ahead of print] PubMed PMID: 2429085.
- Arts HA, Adams ME, Telian SA, et al. Reversible electrocochleographic abnormalities in superior canal dehiscence. Otol Neurotol 2009;30:79Y86.
- Adams ME, Kileny PR, Telian SA, et al. Electrocochleography as a diagnostic and intraoperative adjunct in superior semicircular canal dehiscence syndrome. Otol Neurotol 2011;32:1506Y12.
- Gopen Q, Zhou G, Poe D, et al. Posterior semicircular canal dehiscence: first reported case series. Otol Neurotol 2010; 31:339–44.
- Mikulec AA, Poe DS. Operative management of a posterior semicircular canal dehiscence. Laryngoscope 2006;116:375–8. [PubMed: 16540892].
- Kim HH, Wilson DF. A third mobile window at the cochlear apex. Otolaryngol Head Neck Surg 2006;135:965–6. [PubMed: 17141096].
- Blake DM, Tomovic S, Vazquez A, Lee HJ, Jyung RW. Cochlear-facial dehiscence--a newly described entity. Laryngoscope 2014;124(1):283–9. PubMed PMID: 23712934.
- Agrawal SK, Parnes LS.Human experience with canal plugging. Ann NY Acad Sci 2001;942:300–305.
- Amoodi HA, Makki FM, McNeil M, Bance M. Transmastoid resurfacing of superior semicircular canal dehiscence. Laryngoscope 2011;121(5):1117–23. doi: 10.1002/lary.21398. PubMed PMID: 21520134.
- Teixido M, Seymour PE, Kung B, Sabra O.Transmastoid middle fossa craniotomy repair of superior semicircular canal dehiscenc eusing a soft tissue graft. Otol Neurotol 2011;32:877–81.
- Silverstein H, Kartush JM, Parnes LS, et al.Round window reinforcement for superior canal dehiscence. Otolaryngol Head Neck Surg 2012;147(2suppl):93.

