Vestibular Schwannomas or Acoustic Neuromas by Another Name
Vestibular schwannomas (also known as acoustic neuromas) are benign typically slow growing tumors. They arise from the myelin producing schwann cells which surround the neurons of the vestibular nerve. Myelin produced from the Schwann cells play an integral role in the “insulation” of the neurons which is necessary to enhance electrical conductivity along a nerve. While the term acoustic neuroma has been used interchangeably (usually because the initial presentation generally reflects some element of auditory dysfunction) it appears more pathologically correct that their name should reflects their nerve of origin and histopathology – hence the term vestibular schwannoma (VS).1 Examples of left-sided vestibular schwannomas is seen in Figures 1 and 2.
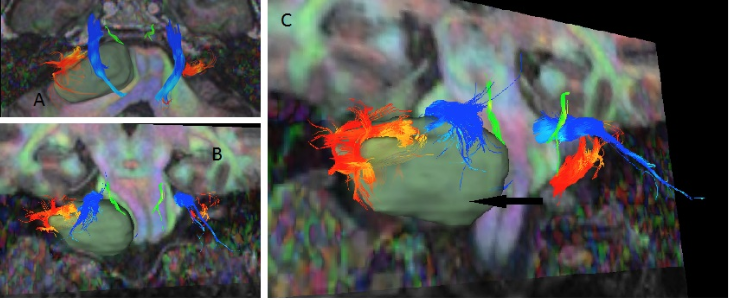
Figure 1. Digital tractography of a left-sided vestibular schwannoma.
Arrow = tumour; green = trochlear nerve; blue = trigeminal nerve; orange = vestibulocochlear nerve.
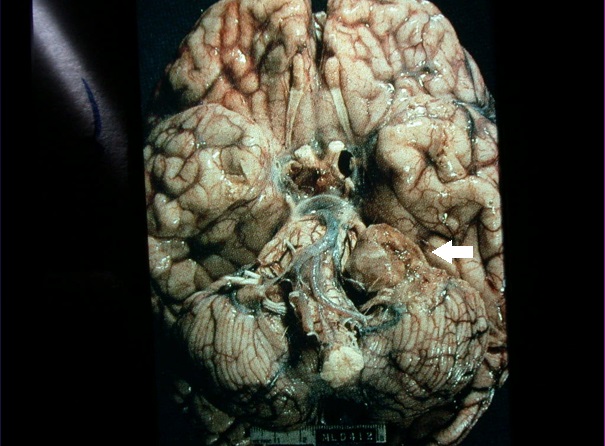
Figure 2. Photo of the posterior fossa of the brain showing a left-sided vestibular schwannoma (arrow).
VS’s represent approximately 5–10% of all intracranial tumours. Their peak incidence occurs between 50–70 years of life. Estimates of their prevalence vary clinically from 13 per million of population to an incidence of 0.57 to 0.87 in post-mortem studies. Professional awareness, increased clinical suspicion and the widespread availability of magnetic resonance imaging (MRI) to a large degree have been responsible for what appears to have been an increasing VS prevalence over the past 40 years.2 An MRI of a right-sided tumour is seen in Figure 3.
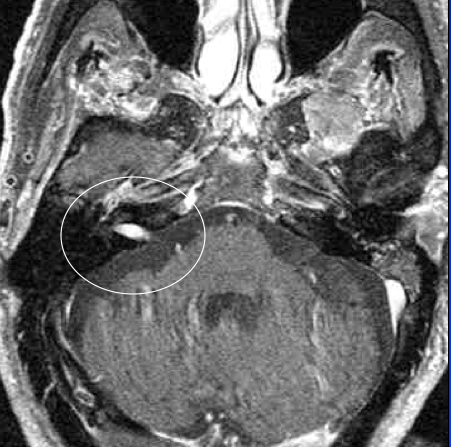
Figure 3. Contrast-enhanced MRI with gadolinium, right-sided tumour.
Histologically most VSs arise within the internal auditory canal (IAC) in an area called the neurolemmal-neuroglia junction (where the central and peripheral myelin sheaths covering the vestibular nerve meet). The tumour is in close approximation to the cochlear and facial nerves within the IAC.
Most VS’s are unilateral but occasionally may bilaterally involve the vestibular nerves. When bilateral involvement is present the condition of neurofibromatosis 2 (NF2) is generally suspected. Both unilateral and bilateral vestibular schwannomas form as a result of a malfunction in part of a gene on chromosome 22 that produces the protein, Merlin which controls the growth of Schwann cells. In NF2 the faulty gene on chromosome 22 is inherited and present in all or most of the somatic cells of the body. For unknown reasons in those with a unilateral schwannoma this gene loses its ability to function properly but is present only in the schwannoma cells on the side involved.1
Natural History
The majority of VS’s grow slowly at a rate of 1–2mm/year. In one 10 year longitudinal prospective study from the University of Toronto, cerebellopontine (CP) angle VS’s grew faster (1.4 mm/yr) than a group of intracanalicular VS’s (0 mm/yr) which seemed to behave in a relatively inert fashion in older individuals (mean age was 61 years at inclusion). Tumours monitored conservatively that eventually required some form of active treatment generally did so within a 5-year timeframe.3
Clinical Presentation
An important axiom to remember is that “an unexplained, progressive unilateral hearing loss and/or tinnitus should be considered a VS until proven otherwise.” Although the tumour arises on the vestibular nerve its slow growth generally allows for the central compensation of any vestibular loss that arises. Apart from symptoms of unilateral auditory dysfunction most patients generally remain relatively asymptomatic until their tumor becomes larger than 3 cm in the CP angle. At this size there can be considerable brainstem and cranial nerve compression that can lead to facial (from trigemminal) and palatal (from glossopharyngeal) numbness from cranial nerve involvement. Compression of the ventricular system can lead to an obstructive hydrocephalus, retinal blindness from papillodema and in the extreme death from unrelenting raised intracranial pressure. Curiously facial weakness is typically a late presentation despite the facial nerve’s proximity to the tumor. It has been argued that motor nerves seem more resilient to the effects of compression than sensory nerves.
Other important clinical observations to consider include factoids that 1–2% of all individuals with a sudden sensorineural hearing loss (SSNHL) are identified to have a VS on MRI scanning. An interesting corollary is that 40% of individuals with a known VS will conversely have a sudden hearing loss at some point during their presentation. Finally the progressive hearing loss does not necessarily appear to correlate well with progressive tumor growth from large scale series primarily from Denmark.2
Diagnosis
Intracranial MRI with gadolinium contrast enhancement is considered the gold standard for diagnosis of a VS. The sensitivity is such that tumors as small as 2–3 mm in diameter can now be routinely diagnosed. Computerized axial tomography (CAT) with contrast (usually an iodinated dye) can also consistently identify tumors that are approximately 5 mm in size or greater in the CP angle. Its sensitivity is not as great for soft tissue resolution as MRI but is sometimes indicated in individuals with a contraindication to MRI (i.e., severe claustrophobia and those with implanted metal devices such as aneurysm clips, cardiac pacemakers, cochlear implants etc.).
The use of different MRI weighting sequences such as T2W and constructive interface in steady state (CISS) can help to minimize the need for gadolinium (i.e., reducing cost and eliminating the remote possibility of a serious complication such as nephrogenic systemic fibrosis). As MRI scanning unlike computerized axial tomography (CAT) scanning does not produce ionizing radiation that could be harmful to the patient if serial frequent scans were needed (especially in those enrolled in a conservative with a “wait and scan” paradigm) (Figure 4).
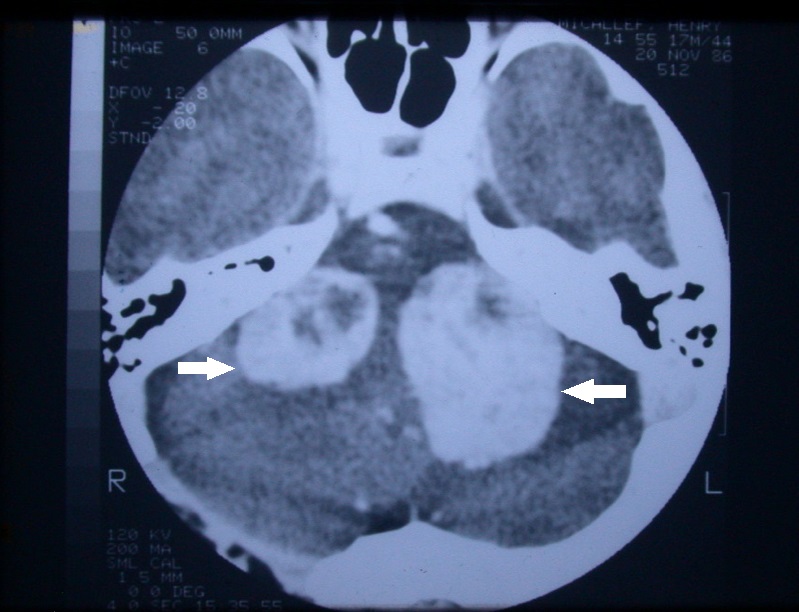
Figure 4. Axial view of a contrast CT scan of a patient with neurofibromatosis type II with bilateral vestibular schwannomas.
The role for auditory brainstem response (ABR) is mentioned primarily in the historic sense. At one time it was an important investigation for determining who would go onto advanced radiologic imaging. While sensitive for selecting individuals whom would be considered candidates for further imaging studies it was prone to false positive results (especially if the cochlear sensorineural reserve was less that 70 dB at 2000 Hz) and false negative results (when tumours were small and did not primarily affect hearing). So called, “stacked” ABR methods have, however, specifically increased the sensitivity in detecting VS’s less than 1 cm initially found on MRI with 95% sensitivity and 88% specificity.4 One prospective study by Cueva compared ABR to the gold standard MRI scan. Asymmetric SNHL was defined as 15 dB or greater asymmetry in two or more frequencies or a 15% or more asymmetry in speech discrimination scores (SDSs). The screening sensitivity of ABR as a screening test was 71% with a specificity of 74% compared to MRI.5 Not surprisingly most centres with readily accessible imaging have abandoned ABR as a screening test for VSs.
Management Options
Management decisions for VS’s is dependant on the size of the tumor, age of the patient, presenting symptoms and patient co-morbidities. The three treatment options currently available include the following:
- Conservative management (wait and scan approach)
- Microsurgical removal (translabyrinthine, retrosigmoid and middle cranial fossa approaches)
- Stereotactic radiotherapy (Gamma knife vs. LINAC).
As most VS schwannomas tend to grow slowly (if they do grow) then a “wait and scan approach” is often undertaken with repeat scans on a 6 monthly to yearly basis. When a tumour, however, becomes greater than 2 cm in size within the CP angle some form of active treatment is generally recommended.
Quality of life studies suggest patients do best when active intervention is not performed. In the prospective series of 72 patients previously described 60% over 10 years did not require active intervention as their tumours did not grow significantly. In the 40% that did require active treatment there was no harm in waiting and there was no change in historically recognized morbidity.3 The downside with the conservative approach is that hearing can continue to deteriorate even in the absence of tumour growth. If there was a window for hearing preservation then it may have been missed.
Microsurgical removal was at one time considered to be the best means of managing an individual with a VS. Once removed it would be unlikely for a tumour to recur.
In translabyrinthine removal the tumor is via a mastoid/labyrinthine approach generally requiring the combined expertise of both ENT surgeons and neurosurgeons. The major advantage of translabyrinthine surgery is early identification of the facial nerve at the distal end of the IAC and the need for less cerebellar retraction for tumor removal. Unfortunately hearing preservation is not possible.
In retrosigmoid removal the tumor is removed via a standard neurosurgical posterior fossa approach and the IAC is exteriorized to reveal the tumor content. In a middle cranial fossa approach the temporal lobe is gently retraction and the IAC is exteriorized superiorly. Both procedures have the possibility to allow for hearing preservation although even in the best of hands there remains only about a 20 % chance that one can maintain hearing at the same level pre-operatively.6 An interoperative photo of a vestibular schwannoma is shown in Figure 5.
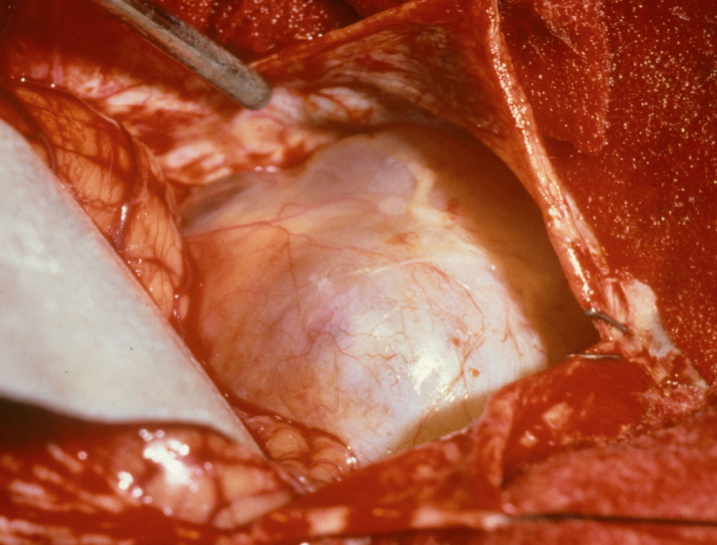
Figure 5. Intraoperative photo of a vestibular schwannoma.
Audiometric and MRI findings appear important in determining whether there is a realistic possibility for hearing preservation. MRI findings of importance for hearing presentation include the length of the tumor-cochlear nerve contact, the degree of distal involvement of tumor in the IAC, tumour size <1.5 cm within the CP angle and the intralabyrinthine signal intensity on CISS weighted studies.
While it is anticipated that a VS can be removed without a complication occurring, complications nevertheless can occur despite best intentions of the surgeons involved. As a general rule the larger the tumour the more likely a complication can occur. This is best demonstrated with facial nerve preservation rates. For example in good hands when a tumour less than 2 cm within the CP angle is totally removed anatomical facial nerve preservation rates (not necessarily the same as functional facial preservation rates) are in the order of 95%. When a tumour is larger than 3 cm in the CP angle anatomical facial nerve preservation rates drop to 60–70% when total tumour removal is attempted. For this reason sometimes a planned surgical decision is made to perform a subtotal removal of tumor leaving some behind intentionally on the facial nerve if it means for better facial function postoperatively. Should any residual VS grow then revision surgery or stereotactic radiotherapy might be considered.
Other complications post-operatively include a 5% chance of CSF leakage with risk for meningitis if the leak does not settle spontaneously. There would also be a 1/1000 chance for injury to the lower cranial nerves at the time of surgery especially if the tumour were large. In the extreme this could affect an individual’s ability to swallow and phonate requiring placement of a feeding tube until swallowing improved significantly. Many individuals also have complaints of dizziness and imbalance post op until compensation for their acute vestibular loss occurs.
In sterotactic radiation the aim is to “biologically sterilize” the tumor that while still present does not grow (or possibly involutes in size). Radiation does this by pathologically inducing an endarteritis obliterans picture where over a number of months or years the tumor is progressively starved of its blood supply. When tumors are < 2 cm in the CP angle, radiation control rates are usually quoted between 85–95%. Tumour control rates generally drop however as a tumours increase in size and there can be a greater chance for radiation injury to the surrounding brainstem and nearby cranial nerves.
Radiation treatment can be delivered in a single setting with the individual’s head in a stereotactic frame (i.e., gamma knife) or in a fractionated fashion (i.e., LINAC) where the individual receives smaller doses of radiation over a number of days or weeks. The theoretic advantage of fractionated stereotactic radiation (FSR) is that it offers both a higher tumour dose and the potential for better sparing of facial and auditory cranial nerve function.
Unfortunately like surgery complications can occur following radiotherapy. Most radiation associated adverse effects, however, occur in a delayed fashion.
With radiation therapy there is a 5% chance of facial weakness (not paralysis) occurring. There would also be a 5% chance of radiation injury to the trigeminal nerve leading to trigeminal neuralgia. Severe headaches from communicating hydrocephalus occurs in approximately 5% of patients. In the extreme if this did not settle then a fluid diversion procedure might be required (i.e., a ventriuloperitoneal [VP] shunt). Whether salvage surgery for recurrent disease would lead to higher complication rates in those where radiation treatment was not effective still remains contentious. In general one would expect gradual progressive worsening of hearing post-radiotherapy even if the tumor did not grow.7 While exceedingly rare the development of radiation induced tumours (benign tumours such as meningiomas and primary brain cancers such as glioblastoma multifomens) have been reported in the past.1
While medical therapy does not play an active role yet in the management of VS’s there has been interest in molecules such as bevacizumab (a neutralizing anti-vascular endothelial growth factor antibody) and imatinib (that affects platelet derived growth factor receptors) in the hopes that they might slow tumour growth.8
Summary
A VS should be considered in any individual with unexplained unilateral hearing loss and tinnitus. To date MRI scanning with gadolinium remains the gold standard for diagnosis. Once identified management options can include a conservative “wait and scan” approach, microsurgical removal or stereotactic radiation. Most tumours overall tend to grow relatively slowly offering the patient a number of treatment options to consider. Treatment decisions need to take into consideration a number of variables such as tumor size, the known natural history of tumor growth, patient age, underlying comorbid health problems and patient preference.
References
- Lunsford DL, Niranjan A, Flickinger JC, Kondziolka D. Navigating change and the acoustic neuroma story: methods, outcomes and myths. Clin Neurosurg 2008;55:47–61.
- Tos M, Stangerup SE, Caye-Thomasen P, et al. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg 2004;130:216–220.
- Hajioff D, Raut VV, Walsh RM, et al. Conservative management of vestibular schwannomas: Third review of a 10 year prospective study. Clin Otolaryngol 2008;33:255–59.
- Don M, Kwong B, Tanaka C, et al. The stacked ABR: A sensitive specific screening tool for detecting small acoustic tumors. Audiol Neurotol 2005;10:274–90.
- Ceuva RA. Auditory brainstem response vs magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope 204;114:1686–92.
- Arriaga MA, Chen DA. Facial function in hearing preservation acoustic neuroma surgery. Arch Otolarynagol Head Neck Surg 2001;127;543–6.
- Regis J, Tamura M, Delsanti C, et al. Hearing preservation in patients with unilateral vestibular schwannoma after gamma knife surgery. Prog Neurol Surg 2008;21:142–51.
- Plotkin SR, Stemmer-Rachamimov AO, Barker FG, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 2009;361:358–67.

