Mysteries of the Hearing Brain
We’re not too old to change: Auditory training and neuroplasticity
I have reached the age at which my physician frequently tells me that my symptoms are due to the aging process, and that I need to figure out a way to live with them. Sometimes it helps to know that there is nothing seriously wrong, but sometimes I would prefer to be offered a solution to my difficulties. I don’t want to be told, “You’re old. Deal with it!” And, I suspect that most of our patients, especially the baby boomers, want real solutions rather than platitudes. Fortunately, evidence regarding brain plasticity offers some hope for creative solutions that are not completely device-focused. In fact, our brains demonstrate a marvelous capacity for plasticity, even into older age. Changes in brain function can have either positive or negative consequences for perception. For example, sensory deprivation results in changes in neural excitability and reorganization of the central regions of the brain, and these changes may result in the perception of tinnitus, the theme of this issue of Canadian Audiologist. Many treatment approaches to tinnitus are based on principles of neuroplasticity, using sound enrichment to reverse changes induced by sensory deprivation.
These same principles might be applied to impaired auditory processing that accompanies age-related hearing loss. After a period of sound deprivation, the use of hearing aids or cochlear implants may induce changes in neural processing. However, the reintroduction of sound stimulation may not restore age-related auditory processing deficits that occur independently of cochlear hearing loss. These deficits may limit outcomes associated with hearing aid or cochlear implant use, despite significant improvements in digital technology. To help to overcome limitations resulting from processing deficits, several software applications have been developed with the aim of improving speech understanding performance, especially in noisy backgrounds. Many of these applications use approaches that purport to strengthen auditory-cognitive connections.
Listening and Communication Enhancement (LACETM, Neurotone, Inc.) and BrainHQ (Posit Science, Inc.) are two examples of software programs that employ auditory-cognitive principles. Studies that evaluated the effects of these programs have had mixed results. LACE training modules target four areas, speech-in-noise, rapid speech, competing speaker, and word memory. A small study of LACE efficacy that included three groups (new HA users plus training, n = 8; experienced HA users plus training, n = 14; and new HA users with no training, n =7) found larger training effects on the QuickSIN scores in the new HA users compared to experienced HA users and control participants.1 However, a larger study conducted in 279 veterans across three centres did not find significant improvement in either speech-in-noise performance or in self-assessment measures in either new or experienced users when compared to participants who received standard-of-care hearing aid intervention services.2 Although LACE targets situations that are typically problematic for older adults, a duration of ten hours (30 minutes per day for 20 sessions) may not be sufficient to engender changes, especially when the number of hours is split among the four modules.
BrainHQ (formerly called Brain Fitness) also uses an auditory-cognitive approach that combines perceptual and cognitive tasks (e.g., processing speed and memory). My dissertation used the original program, Brain Fitness, to determine if this approach leads to improvements in behavioral performance (speech-in-noise, memory, and processing speed) and neural speech representation in older adults. I was particularly interested in Brain Fitness because the auditory training focuses on improving temporal processing through adaptively decreasing the duration of the consonant-vowel transition. Because the consonant transition is perceptually vulnerable in noise,3 I assumed that better encoding of this representation might improve speech-in-noise performance. The training was intense – one hour a day, five days a week, over the course of eight weeks for a total of 40 hours of training. We found that auditory training reduced the frequency-following response amplitude in the group with hearing loss (n=15), but not in the group with normal hearing (n=14).4 In fact, after training, spectral amplitudes in the hearing-impaired group were equivalent to the normal-hearing group (Figure 1). This may seem counterintuitive unless you consider that a decrease in afferent input can actually lead to a compensatory increase in neural firing. This same decrease in spectral amplitude was also found in new hearing aid users after six months of hearing aid use.5 Therefore, the reintroduction of auditory input through hearing aid use or auditory training may have the potential to at least partially reverse maladaptive effects of sensory deprivation. We also found that the auditory training group improved in speech-in-noise performance (QuickSIN) and on measures of memory, attention, and processing speed.
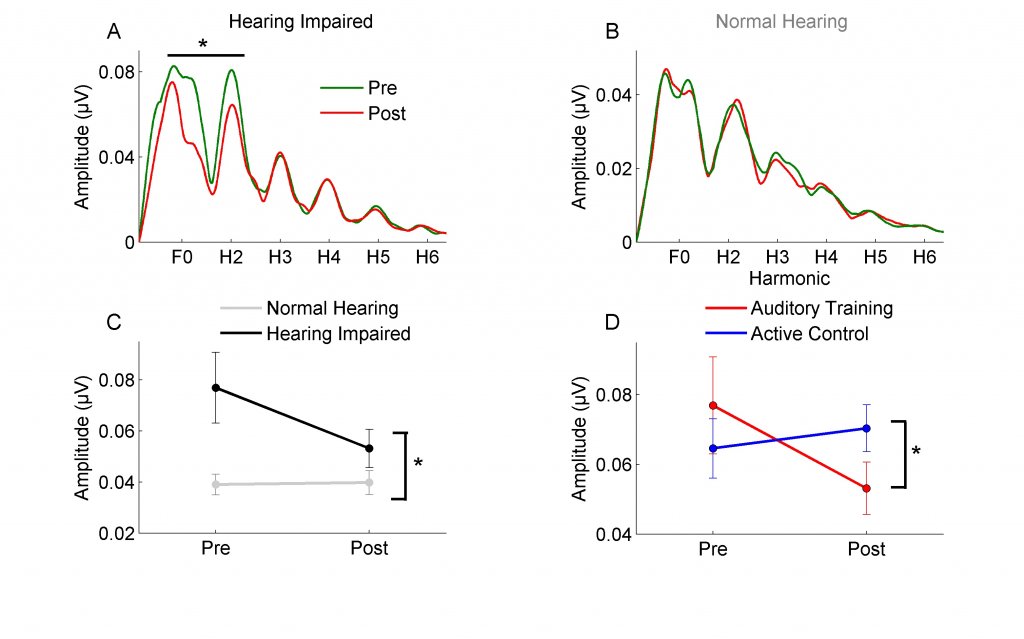
Figure 1. Spectral amplitudes of the fundamental frequency (F0) and the second harmonic (H2) significantly decreased after training in the hearing-impaired group (panel A) but not in the normal-hearing group (panel B). Panel C: Significant differences in spectral amplitude between hearing-impaired and normal-hearing groups at the pre-test session are no longer present at post-test. Panel D: The decrease in spectral amplitude was seen in hearing-impaired participants of the auditory training group but not of the active control group. Adapted with permission from Anderson et al.4
These training effects were not replicated in a study conducted by Saunders et al. in 2018,6 who compared the effectiveness of four interventions in 99 blast-exposed veterans: (1) compensatory communication strategies (CCS), (2) CCS + FM system, (3) CCS + auditory training (AT), and (4) CCS, FM, and AT. They used the Brain Fitness program for the auditory training component. Overall, they found that the FM system combined with CCS was the most beneficial intervention. Their participants had poor compliance with auditory training – only 8.1% of the participants completed at least 30/40 total hours of training. It should be noted that the participants in my dissertation study received compensation for the hours they spent in training, which were monitored online, and therefore we had good compliance. The lack of compliance in the Saunders study speak to the need to provide training that is engaging and intrinsically reinforcing. To that end, new studies are investigating gaming platforms for auditory training with some success.7
In 2017, the National Institute of Aging awarded more than $8 million to the University of Maryland (P.I.: Sandra Gordon-Salant) to develop an innovative approach to improving hearing performance in older adults. The project spans several labs and includes three main projects. Project 1 is examining cortical changes with auditory training in an animal model. Project 2 is examining focused training that is targeted at temporal processing deficits. Project 3 is using an auditory-cognitive approach to evaluate the brain’s ability to form new auditory-cognitive connections. By combining expertise and diverse approaches, we are hoping to develop better solutions for individuals with hearing loss. In future issues, I plan to share with you the results of these projects. As audiology continues to evolve as a profession, I hope that we will be able to offer new, creative solutions to meet our older patients’ needs.
References
- Olson AD, Preminger JE, and Shinn JB. The effect of LACE DVD training in new and experienced hearing aid users. J Am Acad Audiol 2013;24(3):214–30.
- Saunders GH, et al. A randomized control trial: Supplementing hearing aid use with Listening and Communication Enhancement (LACE) auditory training. Ear Hearing 2016.
- Miller GA and Nicely PE. An analysis of perceptual confusions among some English consonants. J Acoust Soc Am 1955;27(2):338–52.
- Anderson S, et al., Training changes processing of speech cues in older adults with hearing loss. Frontiers Syst Neurosci 2013;7(97):1–9.
- Karawani H, Jenkins KA, and Anderson S. Neural and behavioral changes after the use of hearing aids. Clin Neurophysiol, 2018. 129(6): p. 1254-1267.
- Saunders GH, et al. A randomized controlled trial to evaluate approaches to auditory rehabilitation for blast-exposed veterans with normal or near-normal hearing who report hearing problems in difficult listening situations. J Am Acad Audiol 2018;29(1):44–62.
- Whitton JP, et al. Audiomotor perceptual training enhances speech intelligibility in background noise. Curr Biol 2017;27(21):3237–3247.e6.

