Update on cVEMP and oVEMP Testing in Superior Canal Dehiscence
CERVICAL AND OCULAR VEMPS
High intensity auditory signals not only stimulate the cochlea but also activate the vestibular system and can evoke short latency sound evoked reflexes in several muscles including the anterior neck muscles and extraocular muscles. These reflexes can easily be recorded with surface electrodes placed either on the sternocleidomastoid muscle (SCM) or in proximity to the inferior oblique (extraocular) muscle.1–3 The evoked response is referred to as a vestibular evoked myogenic potential (VEMP). A VEMP recorded from the SCM is traditionally referred to as a cervical VEMP (cVEMP; see Figure 1A) and a VEMP recorded from surface electrodes placed beneath the eyes near the inferior oblique has been termed an ocular VEMP (oVEMP; see Figure 1B). A very loud acoustic stimulus (e.g., ~100 dB nHL), bone conduction stimulus, a mechanical head tap, or galvanic stimulation can be used to elicit a VEMP response.
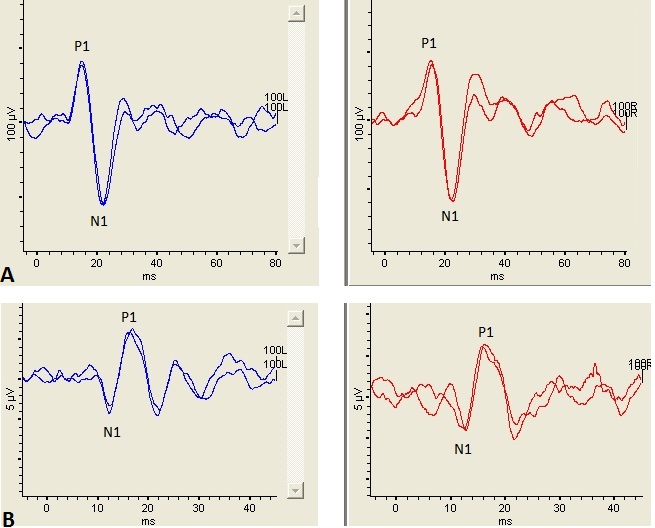
Figure 1. Left panel shows a cVEMP (top) and oVEMP (bottom) recorded from the left ear and right panel shows a cVEMP (top) and oVEMP (bottom) recorded from the right ear. The stimulus was a 500 Hz tone burst presented at 100 dB nHL. (A): Te cVEMP consists of an initial positive peak occurring ~13 msec (P1) followed by a negative peak occurring ~23 msec (N1). ( B): Icontrast to the cVEMP, the oVEMP consists of an initial negative peak occurring ~10 msec (N1) followed by a positive peak occurring ~15 msec (P1). Noe the difference in scaling between the cVEMP and oVEMP waveforms. Th cVEMP is considerably larger than the oVEMP, most likely because the sternocleidomastoid muscleSCM is uch larger muscle than the inferior oblique muscle.
Though a cVEMP and oVEMP response is dependent on the integrity of the entire reflex pathway (i.e., end organ, afferent pathway, central connections, efferent pathway, end muscle), these tests are typically interpreted as an assessment of otolith end organ function. The cVEMP measures the integrity of the saccule and its connections through the inferior vestibular nerve.4 Though the end organ origins of the oVEMP in response to air conduction stimuli are still being debated in the literature, the strongest evidence supports the utricle as being responsible for the oVEMP response.5 Thus, the oVEMP is a measure of utricular and superior vestibular nerve function. Figure 1 illustrates examples of a cVEMP and an oVEMP waveform recorded from a healthy adult.
CLINICAL UTILITY OF CVEMP AND OVEMP
The strongest advantage of cVEMP and oVEMP tests is their ability to measure a different part of the vestibular system (i.e., otolith end organs) than videonystagmography (VNG) and rotational tests which assess the lateral semicircular canal (SCC) and its connections through the superior vestibular nerve. VEMP tests also assess the right and left labyrinth separately, making VEMPs further useful in localizing side of lesion. Another advantage is that both these tests are relatively fast and very tolerable for patients. However, one of the biggest limitations of VEMP tests is that the response rate decreases in older patients. In other words, as individuals age, the rate of bilaterally absent VEMP waveforms increases, even in healthy controls.6,7
One of the clinical limitations of VEMP tests is that, as with most vestibular tests, VEMPs assess site of lesion, not necessarily the presence or absence of disease. For example, cVEMPs are often abnormal in cases of inferior vestibular neuritis, while oVEMPs and caloric testing (i.e., both measures of superior nerve function) are usually normal.8 However, the diagnosis of inferior neuritis is ultimately based on case history. Further, cVEMPs are hypothesized to be useful in Meniere’s disease, another diagnosis based on clinical history and audiometric findings, because the saccule is believed to be the most involved structure in endolymphatic hydrops, following the cochlea. Caloric and rotary chair tests do not assess the saccule and may underestimate vestibular impairments in Meniere’s disease. However, the sensitivity and specificity of cVEMPs are reportedly only 50% and 48.9%, respectively, in patients with unilateral definite Meniere’s disease.9 VEMPs are not essential for the diagnosis of migraine, which is a clinical diagnosis, but it may be important to quantify vestibular involvement. One recent report suggested that VEMPs are bilaterally absent more often in patients with migraine compared to controls.10 Finally, it has been hypothesized that patients with utricle and saccule impairments, as measured using cVEMP and oVEMP tests, may be more susceptible to BPPV since the otoconia responsible for canalithiasis originate in the otolith end organs.11
Despite the limitations, there is at least one vestibular pathology where VEMP tests are extremely useful and, in fact, measure the presence of disease. That is, one of the best uses of the VEMP lies in its ability to help in the diagnosis of superior semicircular canal dehiscence (SCD).
SUPERIOR SEMICIRCULAR CANAL DEHISCENCE
In 1998, Minor and colleagues reported a new vestibular pathology wherein sound and pressure induced vertigo was caused by an absence of bone (i.e., a dehiscence) over the superior SCC beneath the floor of the middle cranial fossa. These patients tended to complain of odd symptoms such as vertigo when sneezing, hearing their eyes move, and sensitivity to loud sounds. The hearing tests of these patients usually showed atypical low frequency air-bone gaps in the presence of normal immittance testing (consequently many of these patients had undergone exploratory middle ear surgery with negative results).12
Dehiscence of the bone overlying the superior SCC is termed superior semicircular canal dehiscence, or SCD. Whereas the cochlear system is comprised of two openings (the oval and round windows) and has relatively low impedance, the vestibular fluids are essentially incompressible, in a healthy system, and minimally affected by sound pressure. In the case of SCD, there exists a “third window” (i.e., the dehiscent bone) which allows sound and pressure changes to displace endolymph within the affected SCC. Patients with SCD often report a cluster of vestibular and auditory symptoms, the most commonly reported shown in Table 1.
Table 1. The Most Common Symptoms and Signs of SCD*
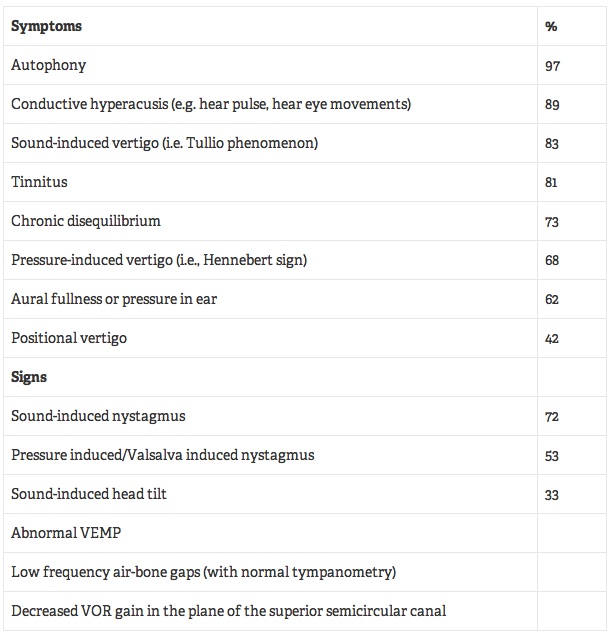
*Percentages, when given, are based on a study of 34 patients with SCD14 and a study of 26 patients with SCD.15 Source: Data from Baloh,13 Tavassolie, et al.,14 and Zhou.15
SCD is typically confirmed using high resolution temporal bone computerized tomography (CT). However, CT scans tend to overestimate the size of the dehiscence and, due to imaging artifacts, can falsely detect dehiscence in patients with a very thin bony covering and in patients who are asymptomatic.14,16 In order to produce an image, raw CT data is filtered with an edge detection filter and noise reduction algorithms are applied, which may actually remove thin bone from a final image resulting in what appears to be a dehiscence.14 This may result in a false-positive and an erroneous diagnosis of SCD. In other words, CT scans have a high sensitivity but can produce false negatives resulting in a low specificity for the diagnosis of SCD.
VEMP tests can be used to detect whether a dehiscence is causing pathological pressure transmission in the vestibular labyrinth, and are therefore uniquely suited for the identification of SCD. In healthy ears, acoustic sounds with a high enough intensity stimulate the saccule and utricle resulting in a muscle reflex we can record using surface electrodes over the muscle of interest (i.e., a VEMP). With a dehiscent canal, the sound pressure causes greater stimulation of the vestibular end organs than would otherwise be expected in a normal functioning labyrinth. The result is often an increase in the amplitude of the VEMP and an abnormally low VEMP threshold. Consequently, both cVEMPs and oVEMPs have been proven useful in assessing the presence of SCD.
VEMP TESTING AND IDENTIFICATION OF SCD: OLD AND NEW CRITERIA
Old Criteria: cVEMP Threshold
cVEMP amplitudes tend to be larger in SCD ears, but the intra-ear variability in cVEMP amplitude precludes it from separating SCD ears from healthy ears. In other words, there is a great deal of overlap in cVEMP amplitude between healthy individuals and those with SCD.17 For this reason, the VEMP parameter of most interest for the identification of SCD has been the cVEMP threshold. Patients with SCD often present with pathologically reduced air and bone conduction cVEMP thresholds.18–22 For example, the average cVEMP threshold using an air conduction tone burst of 500 Hz in a normal ear is around 85–98 dB nHL whereas the average cVEMP threshold in an SCD ear is reportedly between 66 and 81 dB nHL.15,19,20,22 However, one difficulty in using cVEMP threshold to identify SCD is the variability in the definition of “normal” and “pathological.” For example, Zhou et al.15 recommended using a cVEMP threshold cut-off of 65 dB nHL, which reportedly yielded a 91% sensitivity and 95% specificity in a sample of 26 patients with CT confirmed SCD. In a similar study of 21 patients with CT confirmed SCD, Crane et al.23 reported 80% sensitivity and 80% specificity of cVEMP thresholds using a cut-off value of 80 dB nHL. Additionally, Zuniga et al.22 reported that using a higher cut-off value of 85 dB nHL for a 500 Hz air conduction cVEMP resulted in an 86% sensitivity and 90% specificity for the identification of SCD in a sample of 29 adults with surgically confirmed SCD. The ~20 dB nHL range between the recommended cut-off values (i.e., pathological versus normal cVEMP thresholds) can make the significance of the cVEMP threshold difficult to interpret in a clinical setting.
For all intents and purposes, the cVEMP threshold is an excellent diagnostic tool for patients with SCD. However, cVEMP threshold testing can be problematic in that it is time intensive, exhausting to the patient (i.e., patient needs to adequately contract the SCM continuously during the cVEMP recording), potentially exposes the patient to dangerously loud sounds repeatedly, and, as stated above, can be difficult to interpret depending on the cut-off value your lab utilizes.
New Criteria: oVEMP Amplitude and 4000 Hz oVEMP
More recent studies have examined the use of the oVEMP for the detection of SCD.17,22,24–26 Though oVEMP thresholds also tend to be ~10–15 dB nHL lower in SCD patients, oVEMP amplitudes are often tenfold larger in an SCD ear. The most contemporary literature suggests that oVEMP amplitude is a more sensitive test for the detection of SCD compared to oVEMP threshold, cVEMP threshold, and cVEMP amplitude.17,22 In fact, Zuniga et al.,22 using a 105 dB nHL 500 Hz air conduction stimulus, reported 100% sensitivity and 100% specificity of the oVEMP n1-p1 peak-to-peak amplitude with a cut-off value of 17.1 µV or greater. Thus, oVEMP amplitude not only has better response characteristics than cVEMP threshold for the identification of SCD, it can be completed using a single sound intensity thus limiting the patient exposure to multiple runs of intense sound and muscle fatigue.
Manzari et al.24 also sought out a VEMP test parameter that could adequately separate SCD ears from healthy ears using a quick single trial (i.e., avoiding a threshold search). They reported that using a 4000 Hz tone burst at 120 dB SPL for air conduction and 130 dB FL for bone conduction adequately separated all 22 SCD patients from 22 healthy subjects in their study cohort. All 22 SCD patients generated an oVEMP in response to 4000 Hz, whereas not a single one of their 22 healthy control subjects did.
Table 2 summarizes the old and new recommendations for SCD detection using cVEMP and oVEMP tests.
Table 2. Recommended Cut-Off Values, Sensitivity, and Specificity using VEMP Parameters for the Identification of SCD
| Old Criteria | Sensitivity | Specificity |
| cVEMP threshold of 65 dB nHL or less15 | 91.4% | 95.8% |
| cVEMP threshold of 80 dB nHL or less23 | 80% | 80% |
| cVEMP threshold of 85 dB nHL or less22 | 86% | 90% |
| New Criteria | ||
| oVEMP amplitude 17.1 µV or greater22 | 100% | 100% |
| oVEMP present using a 4000 Hz air conduction stimulus24 | 100% | 100% |
Data from: Zhou, et al.,15 Crane et al.,23 Zuniga et al.,22 4Manzari et al.24
Below, we present a case study illustrating the utility of oVEMP testing in the identification of SCD.
CASE STUDY
History
The patient was a 58-year-old woman seen in the Duke Vestibular Lab due to complaints of spinning vertigo, lasting seconds, which occurred when bending over and was accompanied with increased aural pressure in the right ear. She reported a constant fullness in the right ear, exacerbated by bending over, for several years that was extremely bothersome. She also reported chronic disequilibrium for several years. She had been seen by several otolaryngologists but was unable to find a diagnosis for her symptoms.
Audiometry
An audiogram was obtained from an outside facility and showed a mild low-frequency conductive hearing loss at 250 Hz in the right ear and a mild high frequency SNHL bilaterally. Tympanometry showed normal middle ear pressure and compliance and normal equivalent ear canal volumes bilaterally. Acoustic reflexes were present at 1000 Hz bilaterally.
Vestibular Testing
Videonystagmygraphy (VNG) including ocular motility, positional testing, and caloric testing was normal. Rotational testing using sinusoidal harmonic acceleration was normal. cVEMP waveforms in response to a 500 Hz air conduction stimulus presented at 100 dB nHL were present bilaterally and a screening of the cVEMP at 80 dB nHL did not produce a response. oVEMP responses elicited with a 500 Hz air conduction stimulus at 100 dB nHL were absent in the left ear and present in the right ear but with a pathologically large amplitude. oVEMP results are shown in Figure 2. The amplitude of the right oVEMP exceeded 93 µV.
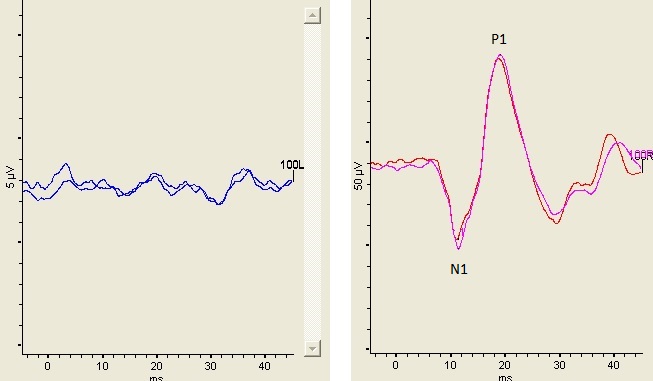
Figure 2. Left panel shows the absence of an oVEMP from the left ear (the patient was over the age of 50 making the absent oVEMP not unusual) and right panel shows the oVEMP waveform elicited from the right ear. The stimulus was an air conduction 500 Hz tone burst presented at 100 dB nHL. The N1 latency was 11.2 msec and the n1-p1 peak-to-peak amplitude was 93.7 µV.
Imaging
Following her vestibular test results, we recommended she undergo a temporal bone CT. The radiologist reported a “bony dehiscent of the superior semicircular canal bilaterally” (see Figure 3).
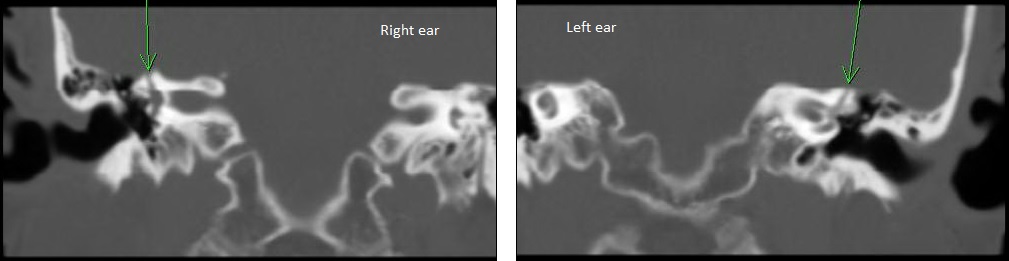
Figure 3. Coronal view of CT scan of patient presenting with right-sided symptoms and abnormal oVEMP on right side. Left panel is right ear and the right panel is the left ear. The arrow was placed by the radiologist onto the image to indicate a possible dehiscence over the superior semicircular canal.
Follow-Up
Though the CT scan identified bilateral SCD, based on symptoms and oVEMP results the patient was ultimately diagnosed with SCD in the right ear only. During follow-up with our otolaryngology colleague, she decided not to undergo surgical treatment but expressed gratitude and relief in finally having a diagnosis and no longer feeling like she was “going crazy.”
DISCUSSION
The above case study shows a patient presenting with bilaterally normal cVEMP thresholds, a pathologically increased oVEMP amplitude in the right ear only, a low frequency conductive hearing loss in the right ear, and a CT scan interpreted as showing a bilateral dehiscence. Additionally, the patient’s symptoms did not include all the “classic” SCD symptoms (see Table 1) that typically trigger the suspicion of SCD (i.e., she denied autophony or dizziness in response to loud sounds). Her major symptoms were aural fullness in the right ear and chronic disequilibrium, which reportedly occur in 62–73% of patients with SCD.14,15 Based solely on her symptoms and normal cVEMP thresholds, a diagnosis of SCD would most likely not have been reached and she would have continued to search for an answer to her symptoms, resulting in an increase in healthcare costs as she sought out new specialists. Conversely, relying solely on her CT report would result in a diagnosis of bilateral SCD, which may have caused an increase in health anxiety for the patient given her lack of symptoms on the left side. Fortunately, the oVEMP was strongly suggestive of right-sided SCD and, given the oVEMP results and her symptoms, she received a diagnosis of right-sided SCD and her anxiety was relieved.
More than likely, CT scans will remain the gold-standard for the identification of SCD. However, based on the possibility of false positives, current research strongly argues that “CT should not be used in isolation for the diagnosis of SCD”14 and that the “low specificity of CT scans creates a risk for over diagnosis of SCD if the coronal CT scans are not correlated with clinical symptoms.”16 VEMP testing provides an excellent and relatively inexpensive test that should be completed prior to exposing the patient to radiation, that may or may not be warranted, or after a positive CT scan to confirm the presence of abnormal pressure transmission prior to the patient undergoing surgery. Further, the most current literature suggests that the oVEMP has a greater sensitivity and specificity for the identification of SCD than the cVEMP. Specifically, oVEMP waveforms with significantly increased amplitudes greater than 17.1 µV or in response to a 4000 Hz stimulus are fast, simple, and objective indicators of SCD that require less effort and time for the patient compared to cVEMP thresholds. Replication studies need to be completed in order to confirm the sensitivity and specificity of the oVEMP amplitude and 4000 Hz oVEMPs for the identification of SCD. However, to date, research suggests the oVEMP is the single most sensitive test in the diagnosis of SCD.
REFERENCES
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 1992;42(8):1635–36.
- Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 2005;116(8):1938–48.
- Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 2007;118(2):381–90.
- Halmagyi GM, Colebatch JG. Vestibular evoked myogenic potentials in the sternomastoid muscle are not of lateral canal origin. Acta oto-laryngologica. Supplementum 1995;520 Pt 1:1–3.
- Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 2010;121(2):132–44.
- Su HC, Huang TW, Young YH, Cheng PW. Aging effect on vestibular evoked myogenic potential. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2004;25(6):977–80.
- Piker EG, Jacobson GP, McCaslin DL, Hood LJ. Normal characteristics of the ocular vestibular evoked myogenic potential. J Am Acad Audiol 2011;22(4):222–30.
- Kim JS, Kim HJ. Inferior vestibular neuritis. J Neurol 2012;259(8):1553–60.
- Egami N, Ushio M, Yamasoba T, Yamaguchi T, Murofushi T, Iwasaki S. The diagnostic value of vestibular evoked myogenic potentials in patients with Meniere's disease. J Vest Res Equilib Orient 2013;23(4–5):249–57.
- Hong SM, Kim SK, Park CH, Lee JH. Vestibular-evoked myogenic potentials in migrainous vertigo. Otolaryngol Head Neck Surg 2011;144(2):284–87.
- Lee JD, Park MK, Lee BD, Lee TK, Sung KB, Park JY. Abnormality of cervical vestibular-evoked myogenic potentials and ocular vestibular-evoked myogenic potentials in patients with recurrent benign paroxysmal postitional vertigo. Acta Oto-Laryngol 2013;133(2):150–53.
- Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surgery 1998;124(3):249–58.
- Baloh RW. Superior semicircular canal dehiscence syndrome: Leaks and squeaks can make you dizzy. Neurology 2004;62(5):684–85.
- Tavassolie TS, Penninger RT, Zuniga MG, Minor LB, Carey JP. Multislice computed tomography in the diagnosis of superior canal dehiscence: how much error, and how to minimize it? Otol Neurotol 2012;33(2):215–22.
- Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol 2007;28(7):920–26.
- Williamson RA, Vrabec JT, Coker NJ, Sandlin M. Coronal computed tomography prevalence of superior semicircular canal dehiscence. Otolaryngol Head Neck Surgery 2003;129(5):481–89.
- Janky KL, Nguyen KD, Welgampola M, Zuniga MG, Carey JP. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol 2013;34(1):127–34.
- Brantberg K, Verrecchia L. Testing vestibular-evoked myogenic potentials with 90-dB clicks is effective in the diagnosis of superior canal dehiscence syndrome. Audiol & Neuro-otol 2009;14(1):54–58.
- Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope 2005;115(10):1717–27.
- Streubel SO, Cremer PD, Carey JP, Weg N, Minor LB. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Oto-laryngol Supplementum 2001;545:41–49.
- Watson SR, Halmagyi GM, Colebatch JG. Vestibular hypersensitivity to sound (Tullio phenomenon): structural and functional assessment. Neurology 2000;54(3):722–28.
- Zuniga MG, Janky KL, Nguyen KD, Welgampola MS, Carey JP. Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol 2013;34(1):121–26.
- Crane BT, Minor LB, Carey JP. Three-dimensional computed tomography of superior canal dehiscence syndrome. Otology Neurotol 2008;29(5):699–705.
- Manzari L, Burgess AM, McGarvie LA, Curthoys IS. An indicator of probable semicircular canal dehiscence: ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol Head Neck Surg 2013;149(1):142–45.
- Rosengren SM, Aw ST, Halmagyi GM, Todd NP, Colebatch JG. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J Neurol Neurosurg Psychiatr 2008;79(5):559–68.
- Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 2008;70(6):464–72.

