Findings from the ACHIEVE RCT: Does hearing care modify dementia risk?
Editor’s Note: Over this past summer, the ACHIEVE study was presented at the Alzheimer’s Association International Conference in Amsterdam, and simultaneously published in the Lancet by Dr. Frank Lin and his colleagues. ACHIEVE stands for Aging and Cognitive Health Evaluation in Elders. As Dr. Kathy Pichora-Fuller reminds us in her column What’s New about Getting Older, publications in the Lancet from several years ago suggested that hearing loss is the greatest POTENTIALLY modifiable mid-life risk factor for dementia. The ACHIEVE study is the first randomized control trial (RCT) to investigate if hearing care can ACTUALLY modify risk for dementia. Overall, the study concluded that there was NO evidence that hearing care reduced risk for dementia. The study is so important that we asked Kathy to write an article in this issue about what ACHIEVE does and does not mean, in addition to her regular column.
Abstract
The long-awaited first results of the Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study were released on July 18, 2023. A brief description of the design and methods of the ACHIEVE study will be provided to highlight how the ACHIEVE study differs from previous research. Notably, it is the first randomized control trial to address the question of whether or not hearing care does modify dementia risk. From these ACHIEVE study results, we gain some answers to questions about WHO could benefit from hearing care for the purpose of reducing the risk of dementia, WHAT kind of hearing care could be beneficial, and WHEN hearing care could be the most beneficial. However, these results also raise new questions regarding WHERE and HOW hearing care should be provided to older adults for whom hearing loss intersects with age-related declines in cognitive health; for example, could hearing care be framed more positively and incorporated into new collaborative inter-professional team models in order to maximize health promotion for older adults more generally? Following a summary of the results, their implications for practice and the need for further research will be discussed.
On July 18, 2023, the long-awaited first results of the Aging and Cognitive Health Evaluation in Elders (ACHIEVE; registered at ClinicalTrials.gov as NCT03243422) study (Deal et al., 2018) were released at the Alzheimer’s Association International Conference in Amsterdam and in a paper published in the Lancet (Lin et al., 2023; see also commentary by Livingston & Costafreda, 2023). From the ACHIEVE study results, we gain some answers to questions about WHO could benefit from hearing care for the purpose of reducing the risk of dementia, WHAT kind of hearing care could be beneficial, and WHEN hearing care could be the most beneficial. However, these results raise new questions regarding WHERE and HOW hearing care should be provided (see column What’s New about Getting Older? in this issue of Canadian Audiologist; Pichora-Fuller, 2023a). How did the ACHIEVE study differ from previous research, what are the answers provided from the preliminary findings, and where do we go from here?
In an earlier article in Canadian Audiologist (Pichora-Fuller, 2023b), the key question posed about hearing intervention and cognition was “Does hearing care modify dementia risk?” At that time, a recent comprehensive review of research (Yeo et al., 2022), suggested that the use of hearing devices might be beneficial for cognitive health. However, there were still many unanswered questions about how the results of the reviewed studies might depend on variations in research designs, especially because none of the studies that had been published were randomized control trials (RCTs). Using the PICO (Population, Intervention, Comparison, Outcome) framework (see ASHA online resources https://www.asha.org/research/ebp/frame-your-clinical-question/), the results could depend on: 1. the characteristics of the populations (P) sampled in the studies (e.g., degree of hearing loss or cognitive status at baseline, or social determinants of health such as sex, race, education, socio-economic position), 2. the interventions (I) administered (hearing aid or cochlear implant and/or other rehabilitation components), 3. the lack of randomization of participants to comparison (C) groups with/without devices and the lack of control for confounding participant characteristics related to help-seeking for hearing loss and other potentially relevant factors (e.g., comorbid health conditions or stigma) that might differ between comparison groups who did or did not use hearing aids, and 4. the outcome (O) measures used to evaluate cognitive change and the time over which change was evaluated. As of a few months ago, hearing health professionals still needed answers to these many lingering questions about the extent to which the results of studies depended on how the research addressed these PICO factors. Answers to these questions are essential to guide the development of evidence-based best practices at the interface of hearing health and cognitive health. The best evidence was expected to come from large-scale prospective RCTs that were carefully designed to address various PICO research considerations. Notably, comparisons between equivalent samples of participants randomly assigned to treatment or control groups would be a critical first step because it is important to show that doing something is better than doing nothing and if there are multiple treatment options then it is important to determine if one of the options is more beneficial. A brief description of how the ACHIEVE RCT addresses PICO factors will be provided, followed by a summary of the results, and a discussion of implications for practice and the need for further research.
PICO Factors in the Research Design and Methods of the ACHIEVE Study
Population: The ACHIEVE study recruited English-speaking, community-dwelling participants who were 70–84 years old with untreated hearing loss and without substantial cognitive impairment or self-reported disability in two or more activities of daily living. Over 3000 people were screened of which 977 were included as participants in the study. In terms of hearing aid candidacy, all participants had adult-onset bilateral hearing loss with a better-ear 4-frequency (0·5–4·0 kHz) pure-tone average (PTA) of 30 to 70 dB HL and at least 60% correct word-recognition in quiet (i.e., people who would be typical first-time hearing aid users). All participants indicated willingness to wear hearing aids on a regular basis. People were excluded if they self-reported permanent conductive hearing loss, a medical condition contra-indicating hearing aid use, or hearing aid use in the last two years. In addition, the Hearing, Handicap Inventory for the Elderly – Screening (HHIE-S; Weinstein, 1986) provided a self-report measure of hearing-related problems in communication functioning (score of 10/40 or greater indicating problems), but the HHIE-S score was not used as an eligibility criterion. Cognitively, all passed screening on the Mini-Mental State Examination (MMSE; Folstein et al., 1975), with inclusion in the study requiring scores ≥25 for those with some college education and scores ≥23 for those with less education (i.e., no indication of probable dementia). All had visual acuity sufficient to read 14-point font.
At each of four geographical locations in the USA (Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MI; Washington County, MD), participants were recruited from two sources: (1) older adults participating in a long-standing observational study of cardiovascular health (Atherosclerosis Risk in Communities; ARIC; Wright et al., 2021), and (2) healthy de novo community volunteers. There were 238 participants recruited from the ARIC study and 739 recruited de novo from the same four communities. The average age was 76.8 (SD = 4.0) years. About half (46%) were female and most (88%) were white.
Notably, there were significant differences in the characteristics of participants recruited from these two sources. At baseline, those recruited from the ARIC study differed significantly from de novo recruits. Specifically, the ARIC recruits were older than the de novo recruits (also more were female and Black), they had more risk factors for cognitive decline (diabetes, hypertension, lower education, living alone), and lower baseline cognitive scores (slightly lower MMSE scores and significantly lower global cognition and cognitive domain factor scores). Thus, those recruited from ARIC had a higher risk for dementia than the de novo group from the outset of the study. Furthermore, the ARIC and de novo recruits had similar PTAs, but de novo recruits had greater self-perceived hearing-related communication functioning as measured by the HHIE-S. Also, note that invitations to participate in ACHIEVE targeted the ARIC participants because they had already participated in research on aging over many decades whereas recruits in the de novo group were people who took the initiative to respond to ads circulated in the community about the ACHIEVE study (i.e., differences in the strategies for inviting the ARIC and de novo recruits to participate may correspond to differences in their history of research experience and the reasons motivating them to engage in research on hearing loss and cognition).
Intervention: Participants were randomly assigned to a hearing intervention (Sanchez et al., 2020) or a control intervention of health education about chronic disease prevention (Newman et al., 2010). Staff at all locations were trained and the implementation of the hearing and education interventions were monitored to ensure consistency. Both interventions were manualized and structured to involve similar degrees of engagement between participants and the staff providing the research interventions, and both groups had follow-up booster sessions every six months for three years.
Those in the hearing intervention had four 1-hour sessions with a study audiologist at intervals of 1-3 weeks after randomization. Bilateral hearing aids fit to target and paired with hearing-assistive technologies (e.g., devices to stream smartphones and television and remote microphones to directly hear other talkers in difficult listening environments) were provided. Training and counselling included systematic orientation and instruction (and re-instruction) on hearing aid use supported by toolkit materials for self-management and communication strategies.
Comparisons: Importantly, half of participants were randomly assigned to the hearing intervention group (N=490) and half to the control health education intervention about prevention of chronic diseases (N=487). The purpose of randomization is to ensure that the key relevant characteristics are equivalent for the participants assigned to the two comparison groups (hearing intervention or education control). Specifically, randomization in the ACHIEVE study was blocked to ensure that both groups included equal numbers of participants according to degree of hearing loss (PTA < 40 or > 40 dB HL), recruitment source (ARIC vs de novo), and geographical site. Randomization was deemed to be successful insofar as there were no obvious differences between participants assigned to the hearing intervention and education control groups. However, as noted above, within the two comparison groups (hearing intervention and education control), there were differences in participant characteristics depending on the source of recruitment (ARIC vs. de novo sub-groups).
Over the 3 years of the study, there were “drop-ins” (16 individuals (16%) in the health education control who bought hearing aids outside of the study) and “drop-outs” (10 individuals (2%) assigned to the hearing intervention group who discontinued hearing aid use entirely). Interestingly, there was a higher drop-in rate for participants in the education control who were recruited de novo (19.4%) than for those recruited from ARIC (7.8%). Analyses considered actual hearing aid use as well as the intended treatment.
Outcomes: The primary outcome measure was change over three years in a global cognition standardized factor score from a comprehensive neurocognitive battery. Cognitive tests included measures of different cognitive domains (some tests used auditory and others visual stimuli; some measured accuracy scores and other outcomes were measured by time or speed). The measures were delayed word recall, digit symbol substitution, incidental learning, trail making parts A and B, logical memory, digit span backwards, Boston naming, word fluency, and animal naming. The HHIE-S was used to measure changes over time in self-reported hearing-related communication functioning. Other outcomes (to be reported in future publications) explored physical, mental and social health with measures that were collected at baseline and annually and also brain MRI scans that were conducted at baseline and in year 3 in half of the participants.
Answers from the Results of the ACHIEVE Study
WHO. Overall, the hearing intervention did NOT reduce 3-year global cognitive decline in the primary analysis of ALL participants in the hearing intervention compared to ALL participants in the education control. These analyses controlled for hearing loss severity (PTA <40 dB vs ≥40 dB HL), recruitment source (ARIC or de novo), geographical site, age, sex, education, and the presence of a genetic marker for Alzheimer’s disease (APOE ε4 alleles).
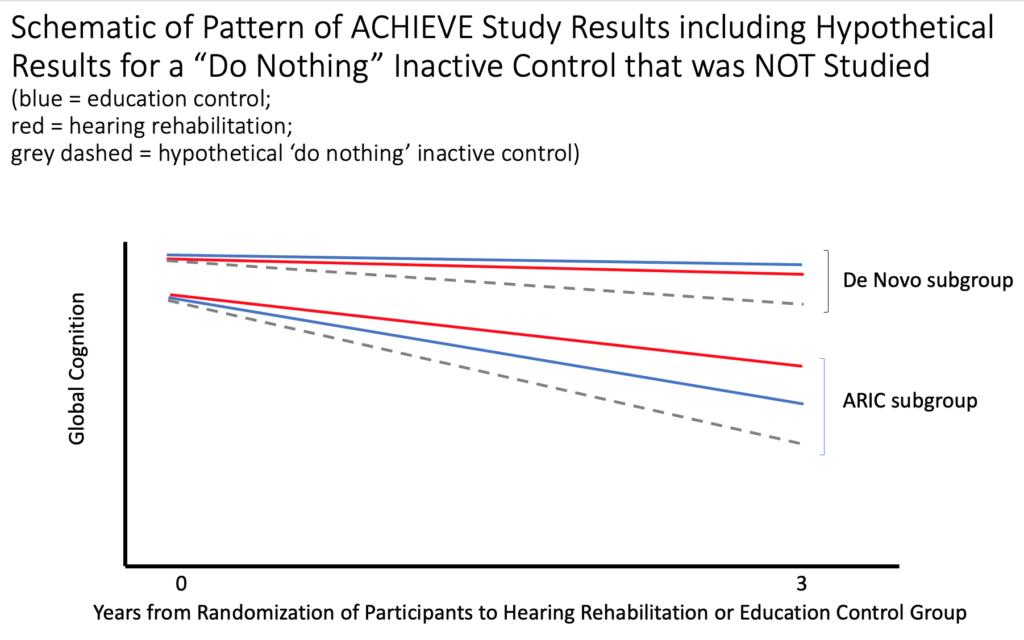
In contrast to the lack of effect observed in analyses of the entire sample, a pre-planned examination of the results for the two sub-groups of recruits (ARIC vs. de novo sources), did reveal a significant benefit from the hearing intervention on 3-year cognitive change in the ARIC sub-group only (see Figure 1 for a schematic of the pattern of results comparing the effects of treatments in the two sub-groups). Importantly, compared to the de novo sub-group, those in the ARIC sub-group had poorer cognitive performance and higher risk for dementia at baseline (i.e., the two sub-groups differed in cognition at the start of the study). For the de novo sub-group (top of figure), who had better cognition and less risk for dementia at baseline compared to the ARIC sub-group, there was relatively little 3-year cognitive decline whether participants were assigned to the hearing intervention group or the education control group (blue and red lines are similar). Considering only those in the ARIC sub-group (bottom of figure), there was a 2.7 greater rate of decline over 3 years for those in the education control group than for those in the hearing intervention group (blue line has a greater slope than the red line). Considering only those who were assigned to the hearing intervention group (red lines for both sub-groups), there was a greater reduction in the slope of 3-year cognitive change in the ARIC sub-group compared to the minimal reduction in the slope observed for those in the de novo sub-group. Essentially, since there was little decline in the de novo group overall, but marked decline in the ARIC group overall, it was possible to observe reduced decline in the ARIC sub-group who were at greater risk of decline, but not in the de novo sub-group had little or no apparent risk of decline over 3 years. Given the overall differences between the ARIC and de novo recruits, the results suggest that hearing intervention might reduce cognitive decline over 3 years in older adults who are already at higher risk for cognitive decline (ARIC sub-group), but not in those with lesser risk for cognitive decline (de novo sub-group).
Figure 1: Schematic Illustrating the Pattern of Results for the De Novo and ARIC sub-groups who were assigned to the education control (blue) or the hearing intervention (red) and hypothetical results for an inactive ‘do nothing’ control that was not studied (grey dashed).
WHAT. Participants receiving the hearing intervention reported a mean of 7.2 (SD = 5.2) hours of daily hearing aid use at 3 years (the hours of hearing aid use were less for those in the de novo sub-group who were ‘drop-ins’). Benefit from the use of hearing aids in the hearing intervention group is suggested by changes over 3 years in self-reported hearing-related communication. Specifically, HHIE-S scores declined from a mean of 15.7 (SD = 10.2) at baseline to 7.8 (7.3) at year 3 for the hearing intervention group, with this change representing a shift in the average score from the problem to the normal range. In contrast, HHIE-S scores increased (worsening of problems) for those in the health education control group from a mean of 14.9 (SD = 9·3) at baseline to 16.2 (9.9) at year 3.
WHEN. Regardless of the source of recruits, hearing intervention resulted in high rates of hearing aid adoption and had positive effects on self-reported hearing-related communication functioning compared to negative effects over 3 years in the education control group. Despite the benefits of hearing intervention in terms of hearing aid usage and reduced self-reported hearing-related communication problems regardless of the source of recruits, the benefits of hearing intervention for reducing cognitive decline did depend on the source of recruits. For the de novo sub-group, there was little cognitive decline over 3 years whether participants were assigned to the hearing intervention group or education control group. For the ARIC sub-group, hearing intervention seemed to protect participants from the relatively rapid decline in cognitive performance observed in peers who were in the education control group. In addition to these differences that depending on WHO received hearing intervention, it seems likely that the results also tell us that WHEN people receive hearing intervention could matter in terms of reducing cognitive decline. It is possible that those in the de novo group (who were younger, had better baseline cognitive performance and fewer risk factors for dementia) might show reduced cognitive decline over a longer time period (i.e., 3 years might be too short a time window). The possible future benefit of hearing intervention in terms of reducing cognitive decline in those at lesser risk of cognitive decline must await the future results of ongoing research on ACHIEVE being conducted over a longer time span (NCT05532657).
Future Research
Some important but yet unanswered questions may be answered by ongoing ACHIEVE research and future analyses (https://www.achievestudy.org/). In particular, we still do not know about the benefits of hearing intervention on incident dementia (i.e., the diagnosis of new cases of dementia) or how the effects of hearing intervention on cognitive performance may be explained in terms of the mechanisms underlying auditory-cognitive associations in aging (for a discussion of these associations see Pichora-Fuller, 2023b).
Dementia Incidence: Audiologists should expect that hearing interventions (not limited to simply buying a hearing aid) could protect those already at higher risk for dementia by reducing their rate of cognitive decline. Note, however, that the effects of hearing intervention on declines in cognitive performance are not the same as preventing dementia. Indeed, no significant effect of hearing intervention on incident cognitive impairment was observed, possibly because there were too few cases for analyses to be meaningful in the 3-year period evaluated. Further research could examine whether there was actually evidence of benefit from hearing intervention on reductions in incident dementia.
Mechanisms. While encouraging, the results do not shed light on the mechanisms underlying the effects of hearing intervention on the rate of decline in cognitive performance for those at higher risk of dementia. Future analyses of brain MRI and social engagement data from the ACHIEVE study will provide important insights into the possible pathways through which hearing intervention might reduce the rate of cognitive decline.
Implications for Practice
WHO, WHAT, WHEN. These first results of the ACHIEVE study answer some who, what and when questions about whether hearing intervention, compared to an educational control, is protective for 3-year cognitive decline. To recap, there was evidence supporting the benefits of hearing intervention in terms of hearing aid usage and improved communication and these benefits were seen regardless of whether participants were recruited from the ARIC study or de novo. In contrast, sub-group differences between ARIC and de novo recruits were observed on the cognitive outcomes, such that those at higher risk for dementia at baseline (the ARIC recruits) showed a reduced rate of cognitive decline over 3 years if they received hearing intervention rather than the education control. For those who had little risk for dementia at baseline (the de novo group), there was no evidence of greater benefit from hearing intervention than from the educational control in 3 years (i.e., there was little cognitive decline in the de novo group no matter which of the interventions they took).
The design of the study was to compare the hearing intervention to an educational control; however, because there was no inactive control group, we do not know if both the hearing intervention and the educational control might have provided benefits compared to doing nothing. Figure 1 also shows hypothetical results for an inactive control. It is possible that both interventions stabilized cognitive performance that might have declined even more if no treatment at all had been provided (grey dashed lines). Both interventions may have provided similar benefits for those in the de novo sub-group who had little risk for dementia. Also, even though the rate of decline for the ARIC sub-group was reduced for those in the hearing intervention group compared to the rate of decline for those in the educational control, it is possible that decline could have been even more rapid for them, because of their higher risk for dementia, if they had not been in the educational control. Thus, we cannot rule out the possible benefits of the educational control compared to doing nothing. Furthermore, we do not know if combining both hearing intervention and health education might be beneficial.
WHERE, HOW. The results of the study were based on interventions administered in four locations using manualized intervention and control protocols. These results raise questions about where and how the hearing intervention could be implemented more generally. The baseline data was collected and in-person hearing intervention and education sessions were completed prior to COVID, but the need for virtual options emerged over the 3 years that ensued until the final outcome data were collected. The need for virtual options during COVID triggered spin-off research that will provide information about variations in how and where similarly effective hearing intervention could be provided (Arnold et al., 2022).
Moreover, whether hearing intervention is provided in-person or online, the ACHIEVE results may trigger important changes in how audiologists collaborate in inter-professional teams with other health professionals involved in providing health care for older adults, including older adults who are at risk for dementia and/or other age-related physical, psychological or social health issues that intersect with hearing and communication functioning (see Pichora-Fuller, 2023a). Notably, the results were released to the broader medical and geriatric communities (respectively, in the Lancet and at the Alzheimer’s Association International Conference) and they were reported in national Canadian media (CBC 2023a,b,c). The overall message conveyed was that hearing interventions are under-used but are beneficial in terms of improving hearing and communication functioning. Now there is evidence that hearing intervention may also reduce the rate of 3-year cognitive decline even if only in those who are at higher risk for dementia. In comparison to new drugs that were also presented at the conference and covered in the media in July, hearing interventions present extremely low medical risk and are relatively inexpensive. Therefore, hearing intervention should be promoted as a key component of integrated person-centered care for older adults. In general, hearing and communication functioning may result in better quality of life and also better quality of health care across many aspects of primary care for older adults. Beyond concern with reducing the risk of dementia, audiologists should look forward to implementing hearing care that positively promotes healthy aging (Blustein et al., 2023) and that becomes woven into the fabric of new approaches to inter-professional primary care.
References
- Arnold, M.L., Schwartz, B., Neil, H., Chisolm, T.H., & Sanchez, V.A. (2022). Feasibility and assessment of a hybrid audiology service delivery model for older adult hearing aid users: A pilot Study. Am J Audiol, 21, 31(3S):892-904. doi: 10.1044/2022_AJA-21-00200.
- Blustein, J., Weinstein, B.E., & Chodosh, J. (2023). Messaging clearly and effectively about hearing loss and increased dementia risk. JAMA Otolaryngol Head Neck Surg. Published online August 24. doi:10.1001/jamaoto.2023.2561
- CBC (2023a). https://www.cbc.ca/news/health/hearing-aids-cognitive-decline-dementia-1.6909911.
- CBC (2023b). https://www.cbc.ca/player/play/2246854723894#:~:text=Hearing%20loss%20has%20been%20strongly%20linked%20with%20cognitive%20decline
- CBC (2023c). https://www.cbc.ca/listen/live-radio/1-13-cross-country-checkup/clip/15999209-ask-me-anything-alzheimers-disease-treatments
- Deal, J. A., Goman, A.M., Albert, M.S., et al. (2018). Hearing treatment for reducing cognitive decline: Design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimers Dementia, 4,499–507. https://doi.org/10.1016/j.trci.2018.08.007
- Folstein, M.F., Folstein, S.E., & McHugh, P.R. (1975). Mini-mental state: A practical method for grading the cognitive state of clients for the clinician. J Psychiatr Res, 12, 189-198. https://doi.org/10.1016/0022-3956(75)90026-6
- Lin, F. R., Pike, J. R., Albert, M. S., Arnold, M., Burgard, S., Chisolm, T., Couper, D., Deal, J. A., Goman, A. M., Glynn, N. W., Gmelin, T., Gravens-Mueller, L., Hayden, K. M., Huang, A. R., Knopman, D., Mitchell, C. M., Mosley, T., Pankow, J. S., Reed, N. S., … Coresh, J. (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. The Lancet (British Edition). https://doi.org/10.1016/S0140-6736(23)01406-X
- Livingston, G., & Costafreda, S. G. (2023). Interventions to prevent dementia should target those at high risk. The Lancet (British Edition). https://doi.org/10.1016/S0140-6736(23)01472-1
- Newman, A. B., Bayles, C. M., Milas, C. N., McTigue, K., Williams, K., Robare, J. F., Taylor, C. A., Albert, S. M., & Kuller, L. H. (2010). The 10 keys to healthy aging: findings from an innovative prevention program in the community. J of Aging and Health, 22(5), 547–566. https://doi.org/10.1177/0898264310363772
- Pichora-Fuller, M.K. (2023a). Adaptation and balance are what matters: The results of the ACHIEVE study prompt rethinking hearing care in integrated person-centered care for older people. Canadian Audiologist, 10(4).
- Pichora-Fuller, M.K. (2023b). Is hearing loss in older adults predictive of later development of dementia and does hearing care modify dementia risk? Canadian Audiologist, 10(1). https://canadianaudiologist.ca/issue/volume-10-issue-1-2023/is-hearing-loss-in-older-adults-predictive-of-later-development-of-dementia-and-does-hearing-care-modify-dementia-risk/
- Sanchez, V.A., Arnold, M.L., Reed, N.S., Oree, P.H., Matthews, C.R., Clock Eddins, A., Lin, F.R., & Chisolm, T.H. (2020). The Hearing Intervention for the Aging and Cognitive Health Evaluation in Elders randomized control trial: Manualization and feasibility study. Ear and Hearing, 41(5),1333-1348. doi: 10.1097/AUD.0000000000000858
- Weinstein, B.E. (1986). Validity of a screening protocol for identifying elderly people with hearing problems. ASHA, 28,41–45.
- Wright JD, Folsom AR, Coresh J, et al. (2021). The ARIC (Atherosclerosis Risk In Communities) study: JACC focus seminar 3/8. J Am Coll Cardiol, 77,2939–59. https://doi.org/10.1016/j.jacc.2021.04.035
- Yeo, B. S. Y., Song, H. J. J. M. D., Toh, E. M. S., Ng, L. S., Ho, C. S. H., Ho, R., et al. (2022). Association of hearing aids and cochlear implants with cognitive decline and dementia: A systematic review and meta-analysis. JAMA Neurology. Published online December 05, 2022. https://doi.org/10.1001/jamaneurol.2022.4427