Mysteries of the Hearing Brain – Ingredients for Effective Auditory Learning
In the last issue of Canadian Audiologist (Vol. 6, Issue 4), I reviewed some of the evidence for the benefits of auditory training in older adults. As I mentioned in the article, the evidence in favor of auditory training is conflicting. One reason for this conflicting evidence may be that we do not yet have a good understanding of the factors that enhance auditory training effects on speech perception, especially in older adults. Neurotransmitters likely play an important role in auditory learning, as they regulate levels of excitability in the nervous system. Researchers have investigated the use of neurotransmitters to facilitate plasticity in the auditory system using animal models. For example, vagus nerve stimulation triggers the release of neurotransmitters (e.g., serotonin and norepinephrine), and pairing vagus nerve stimulation with exposure to tones in rats can improve frequency tuning of auditory cortical neurons that have been disrupted by noise exposure.1 In addition, increasing GABA, an inhibitory neurotransmitter, improves retention of frequency tuning in the rat auditory cortex.2 Most humans, however, would prefer not to be injected with chemicals designed to increase transmission of neurotransmitters! Instead, we could consider activities that naturally increase the release of these neurotransmitters. People usually experience pleasure when listening to music – this pleasure is associated with the release of the neurotransmitter dopamine by activation of the brain’s reward network.3
For these and other reasons, researchers have investigated the use of music as a vehicle for improving auditory skills. Music may be an effective means for improving auditory perception for several reasons. Anirudh Patel suggested the OPERA hypothesis to support the crossover benefits of music training to speech perception.4 This hypothesis proposes that adaptive plasticity in speech processing occurs when five conditions are met: (1) Overlap occurs between anatomical pathways in neural networks that process speech and music, (2) Precision of processing is higher for music than for speech, (3) Emotion is elicited by music, which may engage the brain’s reward network, (4) Repetition through many hours of music practice facilitates auditory learning, and (5) Attention is focused during music activities that engage shared neural networks.
Evidence of the crossover benefits of music training has been observed in young adults for both speech-in-noise perception5 and neural representation of speech stimuli.6 Young adult musicians show lower speech-in-noise thresholds and higher auditory working memory scores than young adult nonmusicians, and musicians’ frequency-following responses (FFRs) to speech syllables show greater resistance to the degrading effects of noise than those of nonmusicians. Young listeners, however, generally do not complain about hearing difficulties, and they can carry on a conversation in a noisy bar with relative ease. A more important demonstration of music’s beneficial effects would be with older adults. Does music training offset the known age-related declines in temporal processing that affect speech-in-noise perception? Music training does appear to have a protective effect against age-related declines in behavioral measures of temporal processing (e.g., gap detection) and speech perception (e.g., speech-in-noise thresholds).7, 8 This behavioral benefit may partially result from the strengthened neural representation of speech. Age-related reductions in neural precision of speech representation have previously been demonstrated in studies that focus on midbrain and cortical processing of speech stimuli.9-11 One example of decreased neural precision is a delay in the latency or timing of waveform peaks in the auditory brainstem response or the FFR. Parbery-Clark et al.12 demonstrated that the typical latency delays observed in older nonmusicians are not observed in older musicians (Figure 1). Note that the age-related changes in neural timing are specific to the onset and consonant transition regions of the response, but not the vowel region. The consonant region of the syllabus is perceptually vulnerable in noise13; therefore, the more precise neural representation of the consonant transition may lead to a better speech-in-noise performance in older musicians.
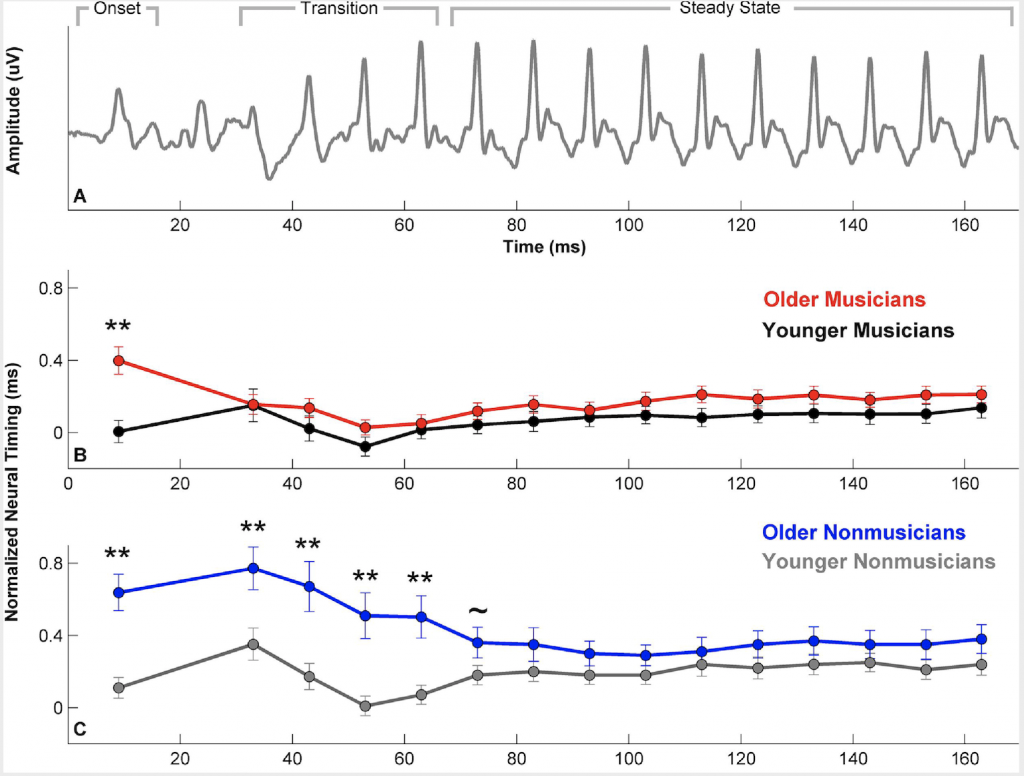
Figure 1. Musicianship offsets typical age-related delays in normal timing. Panel A: Group average frequency-following response (FFR) of young musicians to the speech syllable [da] divided into onset, consonant transition, and steady-state vowel regions. Panels B and C show the timing of individual peaks of the FFR. To facilitate visualization of the peaks on the same time scale, the timing has been normalized relative to average latencies in young adults, such that positive peaks indicate a delay relative to the expected response latency. Modified with permission from Parbery-Clark et al. Neurobiology of Aging 2012.
It should be noted that the older musicians in the Parbery-Clark study had been playing music professionally for most of their lives. Although it may be beneficial to begin music training in older adulthood, the benefits will not be as pronounced as in someone who is a lifelong musician. An alternate approach would be to tailor auditory training based on principles of music training. Playing music is an example of closed-loop sensorimotor training – musicians must monitor subtle changes that they hear and adjust their playing/singing accordingly. This type of closed-loop sensorimotor circuit is also activated during some video game playing. Whitton et al.14 adopted a closed-loop video game approach to an auditory training task. Older adults with hearing loss were trained for eight weeks on a tracking procedure in a virtual soundscape that provided auditory feedback to facilitate the location of the target. In essence, the auditory feedback led the participant to move a stylus/finger to find the hidden object, similar to the way that auditory feedback may lead a violinist to adjust handshape or position to play the desired note. The task was adaptive, in that the background speech babble increased in intensity as performance improved. This training was quite effective – the study participants were able to correctly repeat 25% more words of sentences presented in babble noise.
In summary, auditory training may be an efficacious management recommendation for older adults. The success of this training is likely to be enhanced if it employs techniques known to enhance neuroplasticity, such as the engagement of reward networks or adaptive closed-loop sensorimotor training.
References
- Engineer ND, et al., Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101–104.
- Cisneros-Franco JM., et al. A brain without brakes: reduced inhibition is associated with enhanced but dysregulated plasticity in the aged rat auditory cortex. eNeuro 2018;5(4).
- Zatorre RJ, Musical pleasure and reward: mechanisms and dysfunction. Ann N Y Acad Sci 2015;1337(1):202–211.
- Patel AD. Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Frontier Psychol2011;2:142.
- Parbery-Clark A, et al., Musician enhancement for speech-In-noise. Ear Hear 2009;30(6):653–61.
- Parbery-Clark A, Skoe E, and Kraus N, Musical experience limits the degradative effects of background noise on the neural processing of sound. J Neurosci, 2009;29(45):14100–107.
- Parbery-Clark A, et al. Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. Plos ONE 2011;6(5):e18082.
- Zendel BR and Alain C. Musicians experience less age-related decline in central auditory processing. Psychol Aging 2012;27:410–17.
- Presacco A, et al. Effects of aging on the encoding of dynamic and static components of speech. Ear Hear 2015;36(6):e352–63.
- Roque L, et al. Age effects on neural representation and perception of silence duration cues in speech. J Speech Lang Hear Res 2019;62(4s):1099–16.
- Billings CJ, et al. Electrophysiology and perception of speech in noise in older listeners: Effects of hearing impairment and age. Ear Hearing 2015;36(6):710–22.
- Parbery-Clark A, et al., Musical experience offsets age-related delays in neural timing. Neurobiol Aging 2012;33(7):1483.e1–4.
- Miller GA and Nicely PE. An analysis of perceptual confusions among some English consonants. J Acoust Soc Am 1955;27(2):338–52.
- Whitton JP, et al. Audiomotor perceptual training enhances speech intelligibility in background noise. Curr Biol 2017;27(21):3237–3247.e6.

