Minimally Invasive In-Vivo Functional Ultrasound Imaging of the Auditory Cortex

Recently functional ultrasound imaging has emerged as a new tool for monitoring changes in cerebral blood volume (CBV) associated with neural activity.1–10 Ultrasound’s breakthrough into functional imaging is made possible with recent developments in ultrafast imaging technology. Weakly focused waves insonifying the full field of view are coherently compounded together to create high-quality images with significantly fewer pulses than what had previously been used for B-mode (structural/anatomical) imaging.11–13 The combination of high spatiotemporal resolution as well as low cost and high portability have propped ultrasound to potentially become a ground-breaking imaging modality for functional imaging of the brain. To date, however, studies have been limited to using large conventional ultrasound probes. This has limited the number of practical applications to large craniotomies and thin skull (neonatal) applications. Also, only moderate spatial resolution is possible in the probes used to date. Our research group has extended the possible applicability of functional ultrasound to more common small burr hole surgeries by developing a miniature, very high-resolution ultrasound endoscopic imaging system.14,15 Ironically, this probe was originally developed for imaging the middle ear through the ear canal.16,17 but has now been re-purposed for minimally invasive functional mapping of the auditory response in small animals.The ultrasonic probe used for these studies was an in-house developed 64-element 40 MHz phased array packaged in a 2.5 × 3.1mm endoscopic form factor. The electronic imaging system used was also an in-house developed, fully parallel beamformer capable of data processing rates in the terabits per second. In our preliminary work, rats were anesthetized and subjected to 4, 8, and 15 kHz tones at 97 dB for fixed intervals. These acoustic stimuli were applied to the external ear canal for 2 seconds followed by 2 seconds of baseline measurements, with these measurements repeated across 10 iterations for each tone. The probe was inserted into a small 3 × 6 mm hole for functional imaging of the inferior colliculus (IC). Imaging was undertaken during these intervals by coherently compounding 16 diverging waves at a pulse repetition frequency of 40 kHz on a custom 64-channel firmware and software platform. The effective frame rate was 1 kHz once data transfer to the PC is accounted for. Image acquisition and stimulus were synchronized and automated using a custom Python 2.7 software interface. A standard ultrasound power Doppler algorithm for blood flow detection was used with singular value decomposition filtering to detect the change in blood flow before and during the stimulus.
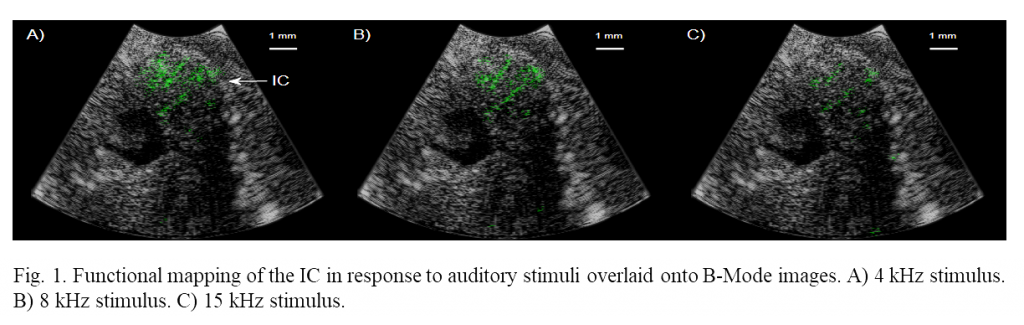
Increases in cerebral blood volume as high as 85% were measured in the IC in response to auditory stimuli to the ear canal. Mapping was performed with very high spatial resolution, 40 and 100 µm of axial and lateral resolution respectively. Figure 1 shows correlations greater than overlaid onto B-Mode images where 4, 8 and 15 kHz tone functional maps are shown respectively. The position of functional activation is in excellent agreement with the anatomical position of the IC. These preliminary studies demonstrate that functional mapping through small burr hole surgeries is possible, vastly increasing the number of potential use-cases for functional ultrasound imaging.
References
- Imbault M, Chauvet D, Gennisson J-L, et al. Intraoperative functional ultrasound imaging of human brain activity. Sci Rep2017;7(1)7304.
- Deffieux T, Demene C, Pernot M, and Tanter M. Functional ultrasound neuroimaging: a review of the preclinical and clinical state of the art. Curr Opin Neurobiol 2018;50:128–35.
- Macé E, Montaldo G, Cohen I, et al. Functional ultrasound imaging of the brain. Nature Method 2011;8(8):662.
- Macé E, Montaldo G, Trenholm S, et al. Whole-brain functional ultrasound imaging reveals brain modules for visuomotor integration. Neuron 2018;100:(5)1241–51.
- Osmanski B-F, Martin C, Montaldo G, et al. Functional ultrasound imaging reveals different odor-evoked patterns of vascular activity in the main olfactory bulb and the anterior piriform cortex. Neuroimage 2014;95:176–84.
- Gesnik M, Blaize K, Deffieux T, et al. 3D Functional Ultrasound Imaging of the cerebral visual system in rodents. NeuroImage 2017;149:267–74.
- Mace E, Montaldo G, Osmanski B-F, et al. Functional ultrasound imaging of the brain: theory and basic principles. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2013;60(3)492–506.
- Bimbard C, Demene C, Girard C, et al. Multi-scale mapping along the auditory hierarchy using high-resolution functional UltraSound in the awake ferret. bioRxiv 2018;249417.
- Demene C, Baranger J, Bernal M, et al. Functional ultrasound imaging of brain activity in human newborns. Sci Translat Med 2017;9(411):6756.
- Demené C, Mairesse J, Baranger J, et al.Ultrafast Doppler for neonatal brain imaging. NeuroImage 2018.
- Montaldo G, Tanter M, Bercoff J, et al. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2009;56(3)489–506.
- Tong L, Gao H, Choi HF, and D’hooge J. Comparison of conventional parallel beamforming with plane wave and diverging wave imaging for cardiac applications: A simulation study. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2012;59(8)1654–63.
- Grondin J, Sayseng V, and Konofagou EE. Cardiac strain imaging with coherent compounding of diverging waves. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2017;64(8)1212–22.
- Bezanson A, Adamon R, Bance M, et al. Fabrication and performance of a miniaturized 64-element high-frequency endoscopic phased array. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2014;6:33–43.
- Samson CA, Bezanson A, Brown JA. A Sub Nyquist, variable sampling, high frequnecy phased array beamformer. IEEE Transact Ultrasonics Ferroelectrics Frequency Control 2017;64(3):568–76.
- Rainsbury J, Landry T, Brown JA, et al. High-frequency ex-vivo ultrasound imaging of the middle ear to show simulated pathology. Otol Neurotol 2016;37(5):586–92.
- Brown JA, Torbatian Z, Adamson R, et al. High-frequency ex-vivo ultrasound imaging of the auditory system. Ultrasound Med Biol 2009;35 :1899-1907, 2009

