Testing for Cochlear Dead Regions: Audiometer Implementation of the TEN(HL) Test
Reprinted with permission from:
https://www.hearingreview.com/practice-building/practice-management/testing-for-cochlear-dead-regions-audiometer-implementation-of-the-tenhl-test
Until recently, the TEN(HL) test for diagnosing dead regions in the cochlea could only be conducted by use of a compact disc player connected to an audiometer. Now, the test has been implemented within the Affinity2.0 and Equinox2.0 PC-based audiometers (version 2.0.4) made by Interacoustics. This article describes: 1) What is meant by a dead region in the cochlea; 2) The basis of the TEN(HL) test for diagnosing dead regions in the cochlea; 3) The implementation of the TEN(HL) test in these audiometers; and 4) The clinical value of diagnosing dead regions.
What Is a Dead Region?
Sounds entering the ear give rise to vibration patterns on the basilar membrane within the cochlea. Each place on the basilar membrane is tuned to respond best to a specific small range of frequencies; high frequency sounds produce maximum vibration toward the base, and low frequency sounds produce maximal vibration toward the apex. The frequency that leads to a maximal vibration at a given place on the basilar membrane is called the characteristic frequency (CF) for that place.
In an ear with normal hearing, the patterns of vibration on the basilar membrane are strongly influenced by the activity of the outer hair cells (OHCs), which are minute sensory cells forming rows along the length of the basilar membrane. The OHCs play a role in what is called the “active mechanism” in the cochlea.1 They do this by changing their stiffness and length in response to the vibrations on the basilar membrane. This activity of the OHCs enhances the response to weak sounds (increasing the amplitude of vibration) and sharpens the tuning on the basilar membrane. This sharpening increases the frequency selectivity of the auditory system (ie, its ability to separate the different frequencies that are present in complex sounds such as speech and music).
The amplified vibrations are then detected by the inner hair cells (IHCs), which form a single row running along the length of the basilar membrane. In response to vibrations on the basilar membrane, the IHCs release neurotransmitter, and this leads to neural activity in the auditory nerve.
Cochlear hearing loss is often associated with damage to the hair cells within the cochlea.1,2 This damage can give rise to raised hearing thresholds (ie, hearing loss as measured by the audiogram) in two main ways:
- OHC damage impairs the active mechanism in the cochlea, resulting in reduced basilar membrane vibration for a given low sound level.3 Hence, the sound level must be greater than normal to give a just-detectable amount of vibration.
- IHC damage can result in less efficient stimulation of the auditory nerve. As a result, the amount of basilar membrane vibration needed to reach the hearing threshold is larger than normal.4
A cochlear hearing loss up to about 55 dBHL may be caused by damage to OHCs alone. A hearing loss greater than 55 dBHL nearly always involves some loss of function of OHCs and IHCs.2 From measurement of the audiogram alone, it is not possible to determine what proportion of the hearing loss is due to OHC damage and what proportion to IHC damage.
In some cases, the IHCs at certain places along the basilar membrane may be completely nonfunctioning. In addition, the auditory neurons making contact with those places may be nonfunctioning. Places with nonfunctioning IHCs and/or neurons have been referred to as “lacunae”5 and “holes in hearing,”6 but I have used the blunt phrase “dead regions.”7-10 This phrase seems to have caught on, although the phrase “dead zones” is also quite common.
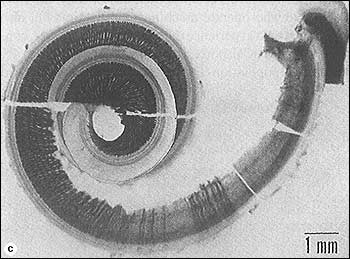
FIGURE 1. Cochlea from a 25-year-old man who had been exposed to gunshots. The dark lines show auditory neurons. There are no neurons coming from the basal part of the cochlea, indicating a dead region.
Figure 1 shows the dissected cochlea of a person who had been exposed to intense impact sounds (gunshots) before dying—in an incident unrelated to gunshots! The dark lines are the neurons that would eventually fuse and form the auditory nerve. There are essentially no neurons coming from the basal part of the cochlea, indicating a high frequency dead region.
Basilar membrane vibration that occurs within a dead region cannot be detected by the neurons connected to that region (if there are any). For example, let’s suppose a case in which the IHCs at the basal (high-frequency) end of the cochlea are nonfunctioning. Neurons connected to the basal end, which would normally have high CFs, will not respond. However, if a high frequency puretone is presented, it may be detected if it produces sufficient basilar membrane vibration at a region closer to the low-frequency apical end. In other words, a high-frequency sound may be detected via neurons that are tuned to lower frequencies. This is sometimes called “off-place listening” or “off-frequency listening.”
Similarly, if there are no functioning IHCs in an apical region of the cochlea, a low-frequency tone may be detected via neurons that are tuned to higher frequencies. Because of this possibility, the “true” hearing loss at a given frequency may be greater than suggested by the audiometric threshold at that frequency. Also, for this reason, dead regions are not easy to diagnose from the puretone audiogram, although a hearing loss greater than 70 dBHL is often associated with a dead region.11,12
A dead region can be characterized in terms of the range of CFs that would normally be associated with that region. In other words, a frequency-to-place map is used to relate the cochlear location at each edge of the dead region to frequency (Figure 2). For example, suppose that the IHCs are nonfunctioning over a region of the basilar membrane having CFs in the range 2500 to 20,000 Hz. One might describe this as a dead region extending from 2500 Hz upwards. The lower edge frequency (expressed in kHz) of the dead region example is 2500 Hz or 2.5 kHz.
Diagnosing Dead Regions in the Cochlea
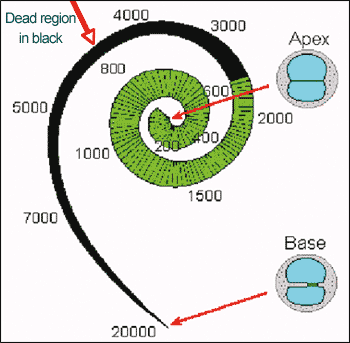
Figure 2. Illustration of how the edge of a dead region can be related to frequency in Hz, using a frequency-to-place map of the cochlea. In this example, the dead region starts at an edge frequency (fe) of about 2500 Hz, and extends upwards toward higher frequencies.
The TEN(HL) test for diagnosing dead regions was designed to be quick and easy to administer and hence suitable for use in clinical practice. The development and validation of the first version of the test are described in Moore et al.8
The test involves measuring the threshold for detecting a puretone presented in a background noise called threshold-equalizing noise (or TEN). The noise was synthesised in such a way that the threshold for detecting a tone in the noise, specified in dBSPL, was approximately the same for all tone frequencies in the range 250 Hz to 10 kHz for people with normal hearing. The masked threshold is approximately equal to the nominal level of the noise specified in dBSPL.
When the puretone signal frequency falls in a dead region, the signal will only be detected when it produces sufficient basilar membrane vibration at a remote region in the cochlea where there are surviving IHCs and neurons. The amount of vibration produced by the tone at this remote region will be less than in the dead region, and so the noise will be very effective in masking it. Thus, the signal threshold is expected to be markedly higher than normal.
Moore et al8 proposed the following rule:
A dead region at a particular frequency is indicated by a masked threshold that is at least 10 dB above the absolute threshold and 10 dB above the nominal noise level.
To make the test easy to administer, the TEN test was recorded on a CD; the noise was on one channel and test tones were on the other channel. For this implementation of the test, the signals from the CD were fed through a two-channel audiometer. The methods used to conduct the test were similar to those used for conventional puretone audiometry, except that the signal threshold was measured in the presence of a continuous background noise.
A problem with this first version of the TEN test was that the clinician had to measure absolute thresholds (audiometric thresholds) twice—once using the tones generated by the audiometer, with level specified in dBHL, and once using the tones from the CD, with level specified in dBSPL. This was inconvenient for the clinician.
To overcome this problem, a second version of the TEN test was developed in which the noise was designed to give equal masked thresholds in dBHL for all frequencies from 500 to 4000 Hz for normally hearing people.13 This version is called the “TEN(HL)” test. As all calibrations were in dBHL, absolute thresholds could be measured either using the tones generated by the audiometer, or using the test tones from the CD; the results were expected to be very similar. This version of the TEN(HL) test can only be used with specific (TDH39, TDH49, and TDH50) headphones.
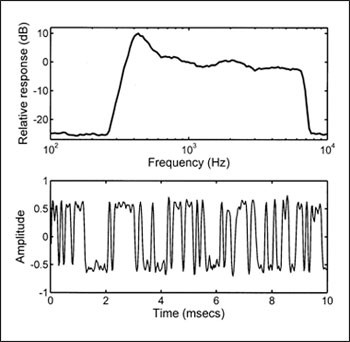
Figure 3. Spectrum (top) and a segment of the waveform (bottom) of the noise used for the TEN(HL) test.
Another advantage of the second version of the test is that the noise has been designed to have minimal amplitude fluctuations—or a type of noise called low-noise noise.14 Figure 3 shows the spectrum of the noise (top) and a sample of the waveform (bottom). Note that all peaks and dips in the waveform are of similar magnitude. This characteristic allows high noise levels to be used without significant distortion being produced by the audiometer or earphone. Hence, testing is possible for more severe hearing losses than could be assessed with the earlier version, without any special equipment being required.
The criteria for diagnosing a dead region are similar to those for the earlier test: a dead region at a particular frequency is indicated by a masked threshold that is at least 10 dB above the absolute threshold and 10 dB above the nominal noise level in dBHL.
Implementation of the Test in Audiometers
The implementation of the TEN(HL) test in the Affinity2.0 and Equinox2.0 PC-based Interacoustics audiometers makes it easy and simple to administer the TEN(HL) test. There is no need to have any equipment external to the audiometer. A pre-defined setup for performing the TEN(HL) test can be selected from a drop-down menu of “User protocols.” One ear at a time is tested, and on-screen boxes are used to determine whether the TEN plus test tone is delivered to the right or left ear. Possible frequencies for the test tone are 500, 750, 1000, 1500, 2000, 3000, and 4000 Hz.
The level of the TEN (in dBHL) can be set using the computer mouse by clicking the appropriate point on the screen, or by using the appropriate knob on the optional dedicated keyboard. Initially, the TEN is turned off while the appropriate level is selected. Some useful rules for selecting the level of the TEN are as follows:
- For frequencies where the hearing loss is less than or equal to 60 dBHL, set the TEN level to 70 dBHL. This is not unpleasantly loud for most people, and it leads to a definitive result.
- When the hearing loss is 70 dBHL or more at a given frequency, set the TEN level 10 dB above the audiometric threshold at that frequency. For example, if the audiometric threshold is 75 dBHL, set the TEN level to 85 dBHL.
- If the TEN is found to be unpleasantly loud, or if the maximum TEN level of 90 dBHL is reached, then the TEN level can be set equal to the audiometric threshold. This should still give a definitive result.
It may be difficult or impossible to apply the TEN(HL) test when the hearing loss at the test frequency is 90 dBHL or more, although it is quite likely that a dead region would be present with such a severe hearing loss. Note that the TEN level does not need to be the same for all test frequencies. Once the level is chosen for a given test frequency, the TEN is turned on continuously, by placing the cursor over “stimulus” or by clicking on “Rev.”
The threshold for detecting a test signal in the TEN is determined using the same procedure as would be used for manual audiometry, except that a step size in level of 2 dB should be used when the tone is in the region of the detection threshold; larger steps can be used initially, to find the approximate threshold. The 2 dB steps are automatically selected when the TEN(HL) test is chosen from the drop-down menu. The use of small steps makes the outcome more precise, and reduces the incidence of false positives.15 Once the threshold has been measured for a given test frequency, the appropriate TEN level is set for the next test frequency, and the process is repeated.
For a person with normal hearing, the threshold of the test tone in the TEN is typically equal to the TEN level. For example, if the TEN level is set to 70 dBHL, the threshold for detecting the test tone is about 70 dBHL for any frequency from 500 to 4000 Hz. If a patient has a cochlear hearing loss but does not have a dead region at the test frequency, then the threshold of the test tone in the TEN is typically a few dB above the TEN level. For example, if the TEN level is set to 70 dBHL, the threshold for detecting the test tone might be 73 dBHL. However, when the test tone frequency falls in a dead region, the threshold for detecting the test tone in the TEN is typically well above the TEN level. The criteria for diagnosing a dead region at a specific frequency are:
- The threshold of the test tone in the TEN is 10 dB or more above the TEN level.
- The threshold of the test tone in the TEN is 10 dB or more above the audiometric (absolute) threshold.
If the TEN level is selected as described earlier, then criterion #2 will automatically be satisfied when criterion #1 is satisfied.
Figure 4 is a screen shot from the Affinity system, showing audiometric thresholds (circles) and TEN(HL)-test results (“TEN” symbols) for a person with good low-frequency hearing but a severe high-frequency hearing loss. The results suggest that this patient has a dead region extending upwards from 1.5 kHz.

Figure 4. Screen shot from the Affinity2.0 system. The open circles show the audiometric (absolute) thresholds. The “TEN” symbols show masked thresholds measured for TEN with a level of 80 dBHL. The TEN(HL)-test criteria for a dead region are met at 1.5, 3, and 4 kHz. The result at 2 kHz is inconclusive, as the masked threshold is not 10 dB or more above the audiometric threshold. In such a case, the result at 2 kHz could be checked using a TEN level of 90 dBHL. Overall, the results suggest that this patient has a dead region extending from 1.5 kHz upwards.
It typically takes about 4 minutes per ear to perform the TEN(HL) test for all test frequencies. In practice, it is usually not necessary to conduct the TEN(HL) test for frequencies where the hearing loss is 50 dB or less. For example, if a patient has a typical sloping hearing loss, with relatively good hearing at low frequencies and poor hearing at high frequencies, it is only necessary to conduct the test for the medium and high frequencies. However, if the patient has an unusually shaped audiogram, such as a localized mid-frequency loss, it may be worth conducting the TEN(HL) test even when the loss is mild.
Clinical Applications of the TEN(HL)
The presence or absence of dead regions can have important implications for fitting hearing aids and for predicting the likely benefit of hearing aids. When a patient has a dead region, there may be little or no benefit in applying amplification (via a hearing aid) for frequencies well inside the dead region. Here are some examples of how knowledge about a dead region can assist the clinician in decision making:
High Frequency Dead Regions
For patients with high frequency dead regions, there may be some benefit in applying amplification for frequencies up to about 1.7fe.16,17 For example, if a patient has a dead region that starts at 1000 Hz and extends upwards from there, there may be some benefit in amplifying frequencies up to 1700 Hz. However, there will probably be no benefit of applying amplification for frequencies above this point. Trying to achieve sufficient gain for frequencies above 1700 Hz might lead to problems with distortion and acoustic feedback. For a patient with an extensive high frequency dead region, a hearing aid incorporating frequency transposition or frequency compression might be a viable option.18,19
Low Frequency Dead Regions
For people with low frequency dead regions, as can occur for example in cases of Ménière’s syndrome, there appears to be some benefit in amplifying frequencies above 0.57fe, but not in amplifying frequencies below 0.57fe.20,21 Amplification of frequencies below 0.57fe can actually lead to reduced speech intelligibility.
Restricted Areas of Cochlear Function
In rare cases, the audiogram may have the form of an inverted V in which hearing is relatively good over a small frequency range, and poor at all remaining high and low frequencies. This can indicate a restricted functioning region in the cochlea, with extensive dead regions below and above it.22-24 However, it is not safe to make a diagnosis of dead regions based solely on an inverted V-shaped audiogram.23 A test such as the TEN(HL) test is needed for a firm diagnosis. For a patient who does have a restricted functioning region—with dead regions above and below the functioning region—the most effective amplification strategy may be to amplify over a limited frequency range around the functioning region.24
Assessing Potential Usefulness of Implants or Hybrids
The TEN(HL) test may also be relevant for patients who are being considered for a cochlear implant. A patient with very extensive dead regions would be likely to do better with a cochlear implant than with a hearing aid (or aids). The test may also be useful for patients considering a combination of a cochlear implant and a hearing aid. Such patients typically have a dead region in the parts of the cochlea that normally respond to medium and high frequencies, but have some functional hearing at lower frequencies. It may be useful to determine the edge frequency (fe) of any dead region. This may be relevant to choosing the most appropriate insertion depth of the electrode array, and to the way that frequencies in the input signal are mapped to acoustic and electric stimulation.25
Some Caveats
The clinician needs to be alert for some special cases. Patients with auditory neuropathy sometimes have high thresholds for detecting the test tone in the TEN, meeting the TEN(HL) test criteria for diagnosis of a dead region even for frequencies where their audiometric thresholds are near normal.26 This does not necessarily indicate that they have extensive dead regions, although they may have only patchy survival of IHCs.27
Patients may also have high thresholds for detecting the test tone in the TEN as a result of central problems (eg, brain injury) in auditory areas resulting from trauma or a stroke.28 These high thresholds may result from poor “detection efficiency” rather than from dead regions. Nevertheless, high thresholds in the TEN are likely in all cases to be associated with a poor ability to understand speech when background sounds are present.
Acknowledgement
The author (with Brian Glasberg and Michael Stone) is the inventor of the TEN(HL) test and has leased the technology to Interacoustics.
References
- Moore BCJ. Cochlear Hearing Loss: Physiological, Psychological and Technical Issues. 2nd ed. Chichester, England: Wiley; 2007.
- Schuknecht HF. Pathology of the Ear. 2nd ed. Philadelphia: Lea and Febiger; 1993.
- Ruggero MA, Rich NC. Furosemide alters organ of Corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11:1057-1067.
- Liberman MC, Dodds LW. Single neuron labeling and chronic cochlea pathology. III. Stereocilia damage and alterations in threshold tuning curves. Hear Res. 1984;16:54-74.
- Troland LT. The psychophysiology of auditory qualities and attributes. J Gen Psych. 1929;2:28-58.
- Shannon RV, Galvin JJ 3rd, Baskent D. Holes in hearing. J Assoc Res Otolaryngol. 2002;3:185-199.
- Moore BCJ, Glasberg BR. A model of loudness perception applied to cochlear hearing loss. Auditory Neurosci. 1997;3:289-311.
- Moore BCJ, Huss M, Vickers DA, Glasberg BR, Alcántara JI. A test for the diagnosis of dead regions in the cochlea. Br J Audiol. 2000;34:205-224.
- Moore BCJ. Dead regions in the cochlea: diagnosis, perceptual consequences, and implications for the fitting of hearing aids. Trends Amplif. 2001;5:1-34.
- Moore BCJ. Dead regions in the cochlea: conceptual foundations, diagnosis and clinical applications. Ear Hear. 2004;25:98-116.
- Aazh H, Moore BCJ. Dead regions in the cochlea at 4 kHz in elderly adults: relation to absolute threshold, steepness of audiogram, and pure tone average. J Am Acad Audiol. 2007;18:96-107.
- Vinay, Moore BCJ. Prevalence of dead regions in subjects with sensorineural hearing loss. Ear Hear. 2007;28:231-241.
- Moore BCJ, Glasberg BR, Stone MA. New version of the TEN test with calibrations in dB HL. Ear Hear. 2004;25:478-487.
- Pumplin J. Low-noise noise. J Acoust Soc Am. 1985;78:100-104.
- Cairns S, Frith R, Munro KJ, Moore BCJ. Repeatability of the TEN(HL) test for detecting cochlear dead regions. Int J Audiol. 2007;46:575-584.
- Vickers DA, Moore BCJ, Baer T. Effects of lowpass filtering on the intelligibility of speech in quiet for people with and without dead regions at high frequencies. J Acoust Soc Am. 2001;110:1164-1175.
- Baer T, Moore BCJ, Kluk K. Effects of lowpass filtering on the intelligibility of speech in noise for people with and without dead regions at high frequencies. J Acoust Soc Am. 2002;112:1133-1144.
- Simpson A, Hersbach AA, McDermott HJ. Improvements in speech perception with an experimental nonlinear frequency compression hearing device. Int J Audiol. 2005;44:281-292.
- Glista D, Scollie S, Bagatto M, Seewald R, Parsa V, Johnson A. Evaluation of nonlinear frequency compression: clinical outcomes. Int J Audiol. 2009;48:632-644.
- Vinay, Moore BCJ. Speech recognition as a function of high-pass filter cutoff frequency for subjects with and without low-frequency cochlear dead regions. J Acoust Soc Am. 2007;122:542-553.
- Vinay, Moore BCJ, Baer T. Speech recognition in noise as a function of highpass-filter cutoff frequency for people with and without low-frequency cochlear dead regions. J Acoust Soc Am. 2008;123:606-609.
- Moore BCJ, Alcántara JI. The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear Hear. 2001;22:268-278.
- Halpin C. The tuning curve in clinical audiology. Am J Audiol. 2002;11:56-64.
- Vinay, Moore BCJ. Psychophysical tuning curves and recognition of highpass and lowpass filtered speech for a person with an inverted V-shaped audiogram. J Acoust Soc Am. 2010. In press.
- Moore BCJ, Glasberg BR, Schlueter A. Detection of dead regions in the cochlea: relevance for combined electric and acoustic stimulation. In: van de Heyning P, Kleine Punte A, eds. Cochlear Implants and Hearing Preservation. Advances in ORL, 67. Basel: Karger; 2009.
- Vinay, Moore BCJ. TEN(HL)-test results and psychophysical tuning curves for subjects with auditory neuropathy. Int J Audiol. 2007;46:39-46.
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9:1-43.
- Langenbeck B. Textbook of Practical Audiometry. London: Edward Arnold; 1965.

